Prostate specific antigen for early detection of prostate cancer: longitudinal study
- PMID: 19778969
- PMCID: PMC2751815
- DOI: 10.1136/bmj.b3537
Prostate specific antigen for early detection of prostate cancer: longitudinal study
Abstract
Objective: To evaluate if prostate specific antigen test attains validity standards required for screening in view of recent prostate cancer screening trial results.
Design: Case-control study nested in longitudinal cohort.
Setting: Västerbotten Intervention Project cohort, Umeå, Sweden.
Participants: 540 cases and 1034 controls matched for age and date of blood draw.
Main outcome measure: Validity of prostate specific antigen for prediction of subsequent prostate cancer diagnosis by record linkage to cancer registry.
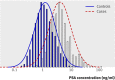
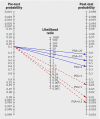
Results: Blood samples were drawn on average 7.1 (SD 3.7) years before diagnosis. The area under the curve for prostate specific antigen was 0.84 (95% confidence interval 0.82 to 0.86). At prostate specific antigen cut-off values of 3, 4, and 5 ng/ml, sensitivity estimates were 59%, 44%, and 33%, and specificity estimates were 87%, 92%, and 95%. The positive likelihood ratio commonly considered to "rule in disease" is 10; in this study the positive likelihood ratios were 4.5, 5.5, and 6.4 for prostate specific antigen cut-off values of 3, 4, and 5 ng/ml. The negative likelihood ratio commonly considered to "rule out disease" is 0.1; in this study the negative likelihood ratios were 0.47, 0.61, and 0.70 for prostate specific antigen cut-off values of 3, 4, and 5 ng/ml. For a cut-off of 1.0 ng/ml, the negative likelihood ratio was 0.08.
Conclusions: No single cut-off value for prostate specific antigen concentration attained likelihood ratios formally required for a screening test. Prostate specific antigen concentrations below 1.0 ng/ml virtually ruled out a prostate cancer diagnosis during the follow-up. Additional biomarkers for early detection of prostate cancer are needed before population based screening for prostate cancer should be introduced.
Conflict of interest statement
Conflicts of interest: None declared.
Figures
Comment in
-
Prostate specific antigen for detecting early prostate cancer.BMJ. 2009 Sep 24;339:b3572. doi: 10.1136/bmj.b3572. BMJ. 2009. PMID: 19778970 No abstract available.
-
Prostate specific antigen. Prostate screening in France.BMJ. 2009 Oct 21;339:b4285. doi: 10.1136/bmj.b4285. BMJ. 2009. PMID: 19846492 No abstract available.
-
PSA and prostate cancer. More research is not needed.BMJ. 2009 Dec 10;339:b5336. doi: 10.1136/sbmj.b5336. BMJ. 2009. PMID: 20007232 No abstract available.
References
-
- Ross KS, Carter HB, Pearson JD, Guess HA. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA 2000;284:1399-405. - PubMed
-
- Yao SL, Lu-Yao G. Interval after prostate specific antigen testing and subsequent risk of incurable prostate cancer. J Urol 2001;166:861-5. - PubMed
-
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin 2008;58:161-79. - PubMed
-
- Lieberman R. Evidence-based medical perspectives: the evolving role of PSA for early detection, monitoring of treatment response, and as a surrogate end point of efficacy for interventions in men with different clinical risk states for the prevention and progression of prostate cancer. Am J Ther 2004;11:501-6. - PubMed
-
- Stenman UH, Abrahamsson PA, Aus G, Lilja H, Bangma C, Hamdy FC, et al. Prognostic value of serum markers for prostate cancer. Scand J Urol Nephrol Suppl 2005;216:64-81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
