p73 is essential for vitamin D-mediated osteoblastic differentiation
- PMID: 19779497
- PMCID: PMC2822032
- DOI: 10.1038/cdd.2009.135
p73 is essential for vitamin D-mediated osteoblastic differentiation
Abstract
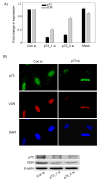
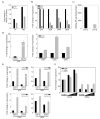
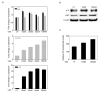
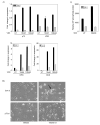
The secosteroid hormone vitamin D3 (VD3) exerts its biological actions through its cognate receptor, the vitamin D receptor (VDR). Vitamin D3 and VDR have a key function in bone formation and keratinocyte differentiation, exert antiproliferative actions in human cancer, and is widely used as a chemotherapeutic agent for cancer. In addition, VD3 promotes differentiation of human osteosarcoma cells by up-regulating genes involved in cell cycle arrest and osteoblastic differentiation. Although considerable work has been carried out in understanding the molecular mechanisms underlying the VD3-mediated differentiation of human osteosarcoma cells, the upstream regulation of VD3 signaling pathway is still unclear. In this study, we show that p73 acts as an upstream regulator of VD3-mediated osteoblastic differentiation. Transcription factor p73, a p53 homolog, has been shown to have a function in development and recently been termed as a tumor suppressor. Silencing p73 results in a significant reduction of VD3-mediated osteoblastic differentiation; although DNA damage induced p73 leads to an increase in VD3-mediated differentiation of osteosarcoma cells. Together, our data implicate a novel function for p73 in vitamin D-mediated differentiation of human osteosarcoma cells.
Figures


References
-
- Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134(12 Suppl):3472S–3478S. - PubMed
-
- Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr Rev. 1989;10(3):294–307. - PubMed
-
- Nezbedova P, Brtko J. 1alpha,25-dihydroxyvitamin D3 inducible transcription factor and its role in the vitamin D action. Endocr Regul. 2004;38(1):29–38. - PubMed
-
- Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10(5):735–48. - PubMed
-
- Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1-5):156–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

