An RNA transport system in Candida albicans regulates hyphal morphology and invasive growth
- PMID: 19779551
- PMCID: PMC2739428
- DOI: 10.1371/journal.pgen.1000664
An RNA transport system in Candida albicans regulates hyphal morphology and invasive growth
Erratum in
- PLoS Genet. 2009 Oct;5(10). doi: 10.1371/annotation/17eb3a67-8f49-454b-acbf-5ff2872c27ff doi: 10.1371/annotation/17eb3a67-8f49-454b-acbf-5ff2872c27ff
Abstract
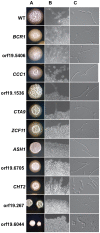
Localization of specific mRNAs is an important mechanism through which cells achieve polarity and direct asymmetric growth. Based on a framework established in Saccharomyces cerevisiae, we describe a She3-dependent RNA transport system in Candida albicans, a fungal pathogen of humans that grows as both budding (yeast) and filamentous (hyphal and pseudohyphal) forms. We identify a set of 40 mRNAs that are selectively transported to the buds of yeast-form cells and to the tips of hyphae, and we show that many of the genes encoded by these mRNAs contribute to hyphal development, as does the transport system itself. Although the basic system of mRNA transport is conserved between S. cerevisiae and C. albicans, we find that the cargo mRNAs have diverged considerably, implying that specific mRNAs can easily move in and out of transport control over evolutionary timescales. The differences in mRNA cargos likely reflect the distinct selective pressures acting on the two species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Johnstone O, Lasko P. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet. 2001;35:365–406. - PubMed
-
- King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol Cell. 2005;97:19–33. - PubMed
-
- Mowry KL, Cote CA. RNA sorting in Xenopus oocytes and embryos. FASEB J. 1999;13:435–445. - PubMed
-
- St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–375. - PubMed
-
- Tekotte H, Davis I. Intracellular mRNA localization: motors move messages. Trends Genet. 2002;18:636–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

