Bacillus anthracis lethal toxin disrupts TCR signaling in CD1d-restricted NKT cells leading to functional anergy
- PMID: 19779559
- PMCID: PMC2742733
- DOI: 10.1371/journal.ppat.1000588
Bacillus anthracis lethal toxin disrupts TCR signaling in CD1d-restricted NKT cells leading to functional anergy
Erratum in
- PLoS Pathog. 2009 Oct;5(10) doi: 10.1371/annotation/cfc9c388-05fa-4b1c-8c5f-99835278a458
Abstract
Exogenous CD1d-binding glycolipid (alpha-Galactosylceramide, alpha-GC) stimulates TCR signaling and activation of type-1 natural killer-like T (NKT) cells. Activated NKT cells play a central role in the regulation of adaptive and protective immune responses against pathogens and tumors. In the present study, we tested the effect of Bacillus anthracis lethal toxin (LT) on NKT cells both in vivo and in vitro. LT is a binary toxin known to suppress host immune responses during anthrax disease and intoxicates cells by protective antigen (PA)-mediated intracellular delivery of lethal factor (LF), a potent metalloprotease. We observed that NKT cells expressed anthrax toxin receptors (CMG-2 and TEM-8) and bound more PA than other immune cell types. A sub-lethal dose of LT administered in vivo in C57BL/6 mice decreased expression of the activation receptor NKG2D by NKT cells but not by NK cells. The in vivo administration of LT led to decreased TCR-induced cytokine secretion but did not affect TCR expression. Further analysis revealed LT-dependent inhibition of TCR-stimulated MAP kinase signaling in NKT cells attributable to LT cleavage of the MAP kinase kinase MEK-2. We propose that Bacillus anthracis-derived LT causes a novel form of functional anergy in NKT cells and therefore has potential for contributing to immune evasion by the pathogen.
Conflict of interest statement
The authors have declared that no competing interests exist.
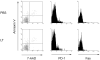
Figures
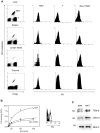

References
-
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. - PubMed
-
- Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

