Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages
- PMID: 19779568
- PMCID: PMC2743285
- DOI: 10.1371/journal.ppat.1000591
Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages
Abstract
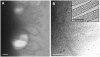
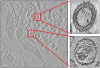
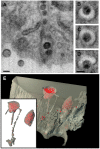
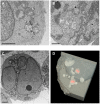
HIV-1-containing internal compartments are readily detected in images of thin sections from infected cells using conventional transmission electron microscopy, but the origin, connectivity, and 3D distribution of these compartments has remained controversial. Here, we report the 3D distribution of viruses in HIV-1-infected primary human macrophages using cryo-electron tomography and ion-abrasion scanning electron microscopy (IA-SEM), a recently developed approach for nanoscale 3D imaging of whole cells. Using IA-SEM, we show the presence of an extensive network of HIV-1-containing tubular compartments in infected macrophages, with diameters of approximately 150-200 nm, and lengths of up to approximately 5 microm that extend to the cell surface from vesicular compartments that contain assembling HIV-1 virions. These types of surface-connected tubular compartments are not observed in T cells infected with the 29/31 KE Gag-matrix mutant where the virus is targeted to multi-vesicular bodies and released into the extracellular medium. IA-SEM imaging also allows visualization of large sheet-like structures that extend outward from the surfaces of macrophages, which may bend and fold back to allow continual creation of viral compartments and virion-lined channels. This potential mechanism for efficient virus trafficking between the cell surface and interior may represent a subversion of pre-existing vesicular machinery for antigen capture, processing, sequestration, and presentation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Orenstein JM. Isn't a picture still worth a thousand words? Ultrastruct Pathol. 2000;24:67–74. - PubMed
-
- Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

