Stem cell platforms for regenerative medicine
- PMID: 19779576
- PMCID: PMC2749253
- DOI: 10.1111/j.1752-8062.2009.00096.x
Stem cell platforms for regenerative medicine
Abstract
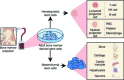
The pandemic of chronic degenerative diseases associated with aging demographics mandates development of effective approaches for tissue repair. As diverse stem cells directly contribute to innate healing, the capacity for de novo tissue reconstruction harbors a promising role for regenerative medicine. Indeed, a spectrum of natural stem cell sources ranging from embryonic to adult progenitors has been recently identified with unique characteristics for regeneration. The accessibility and applicability of the regenerative armamentarium has been further expanded with stem cells engineered by nuclear reprogramming. Through strategies of replacement to implant functional tissues, regeneration to transplant progenitor cells or rejuvenation to activate endogenous self-repair mechanisms, the overarching goal of regenerative medicine is to translate stem cell platforms into practice and achieve cures for diseases limited to palliative interventions. Harnessing the full potential of each platform will optimize matching stem cell-based biologics with the disease-specific niche environment of individual patients to maximize the quality of long-term management, while minimizing the needs for adjunctive therapy. Emerging discovery science with feedback from clinical translation is therefore poised to transform medicine offering safe and effective stem cell biotherapeutics to enable personalized solutions for incurable diseases.
Keywords: adult; allogeneic; autologous; bioengineered; embryonic; immune response; perinatal.
Figures



References
-
- Rossant J. Stem cells and early lineage development. Cell. 2008; 132(4): 527–531. - PubMed
-
- Daley GQ, Scadden DT. Prospects for stem cell‐based therapy. Cell. 2008; 132(4): 544– 548. - PubMed
-
- Rosenthal N. Prometheus's vulture and the stem‐cell promise. N Engl J Med. 2003; 349(3): 267–274. - PubMed
-
- Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov. 2008; 7(2): 131–142. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

