Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1
- PMID: 19779610
- PMCID: PMC2744928
- DOI: 10.1371/journal.pone.0007140
Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1
Abstract
Apoptosis depends upon the activation of intracellular caspases which are classically induced by either an intrinsic (mitochondrial based) or extrinsic (cytokine) pathway. However, in the process of explaining how endotoxin activated monocytes are able to induce apoptosis of vascular smooth muscle cells when co-cultured, we uncovered a transcellular apoptosis inducing pathway that utilizes caspase-1 containing microvesicles. Endotoxin stimulated monocytes induce the cell death of VSMCs but this activity is found in 100,000 g pellets of cell free supernatants of these monocytes. This activity is not a direct effect of endotoxin, and is inhibited by the caspase-1 inhibitor YVADcmk but not by inhibitors of Fas-L, IL-1beta and IL-18. Importantly, the apoptosis inducing activity co-purifies with 100 nm sized microvesicles as determined by TEM of the pellets. These microvesicles contain caspase-1 and caspase-1 encapsulation is required since disruption of microvesicular integrity destroys the apoptotic activity but not the caspase-1 enzymatic activity. Thus, monocytes are capable of delivering a cell death message which depends upon the release of microvesicles containing functional caspase-1. This transcellular apoptosis induction pathway describes a novel pathway for inflammation induced programmed cell death.
Conflict of interest statement
Figures
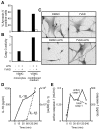

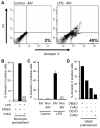
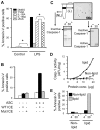
 ) VSMC+ MV from LPS/YVAD monocytes; (
) VSMC+ MV from LPS/YVAD monocytes; ( ) VSMC treated with IL-1RA+ MV from LPS treated monocytes; (□) VSMC treated with IL-18 bp + MV from LPS treated monocytes; (
) VSMC treated with IL-1RA+ MV from LPS treated monocytes; (□) VSMC treated with IL-18 bp + MV from LPS treated monocytes; ( ) VSMC treated with sFAS+ MV from LPS treated monocytes. Apoptosis of VSMC was measured using Annexin V/PI assay. Graph represents average of n = 2 experiments. B) HEK-293 cells were transfected with either wild type or mutant caspase-1 (0.2 µg) along with ASC (0.2 µg) using Lipofectamine 2000. Transfection was normalized using vector controls. Supernatants were collected from the transfected cells and subjected to VSMC. Apoptosis was measured using Annexin V/PI assays. THP-1 cells were lysed and cell lysates were either kept at 4°C or incubated at 30°C for 1 h. Cell lysates were then centrifuged at 15,000 X g for 20 min. Both lipid and non-lipid fractions were measured for caspase-1 activity using C) immunoblot and D) WEHD-enzymatic assay. E) Both fractions were also subjected to VSMC and cell death was analyzed by Annexin V/PI assays.
) VSMC treated with sFAS+ MV from LPS treated monocytes. Apoptosis of VSMC was measured using Annexin V/PI assay. Graph represents average of n = 2 experiments. B) HEK-293 cells were transfected with either wild type or mutant caspase-1 (0.2 µg) along with ASC (0.2 µg) using Lipofectamine 2000. Transfection was normalized using vector controls. Supernatants were collected from the transfected cells and subjected to VSMC. Apoptosis was measured using Annexin V/PI assays. THP-1 cells were lysed and cell lysates were either kept at 4°C or incubated at 30°C for 1 h. Cell lysates were then centrifuged at 15,000 X g for 20 min. Both lipid and non-lipid fractions were measured for caspase-1 activity using C) immunoblot and D) WEHD-enzymatic assay. E) Both fractions were also subjected to VSMC and cell death was analyzed by Annexin V/PI assays.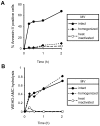
Similar articles
-
Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis.PLoS One. 2014 Mar 18;9(3):e90968. doi: 10.1371/journal.pone.0090968. eCollection 2014. PLoS One. 2014. PMID: 24643116 Free PMC article.
-
Cross talk between vascular smooth muscle cells and monocytes through interleukin-1β/interleukin-18 signaling promotes vein graft thickening.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):2001-11. doi: 10.1161/ATVBAHA.113.303145. Epub 2014 Jul 10. Arterioscler Thromb Vasc Biol. 2014. PMID: 25012128
-
Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage.Atherosclerosis. 2002 Mar;161(1):143-51. doi: 10.1016/s0021-9150(01)00631-1. Atherosclerosis. 2002. PMID: 11882326
-
Cytosolic, autocrine alpha-1 proteinase inhibitor (A1PI) inhibits caspase-1 and blocks IL-1β dependent cytokine release in monocytes.PLoS One. 2012;7(11):e51078. doi: 10.1371/journal.pone.0051078. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226468 Free PMC article.
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
Cited by
-
Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction.Int J Mol Sci. 2018 Oct 29;19(11):3383. doi: 10.3390/ijms19113383. Int J Mol Sci. 2018. PMID: 30380647 Free PMC article. Review.
-
Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease.JACC Basic Transl Sci. 2017 Dec 25;2(6):790-807. doi: 10.1016/j.jacbts.2017.08.004. eCollection 2017 Dec. JACC Basic Transl Sci. 2017. PMID: 30062186 Free PMC article. Review.
-
Role of Extracellular Vesicles in Autoimmune Pathogenesis.Front Immunol. 2020 Sep 23;11:579043. doi: 10.3389/fimmu.2020.579043. eCollection 2020. Front Immunol. 2020. PMID: 33072123 Free PMC article. Review.
-
Engineered extracellular vesicles-like biomimetic nanoparticles as an emerging platform for targeted cancer therapy.J Nanobiotechnology. 2023 Aug 22;21(1):287. doi: 10.1186/s12951-023-02064-1. J Nanobiotechnology. 2023. PMID: 37608298 Free PMC article. Review.
-
Biological properties of extracellular vesicles and their physiological functions.J Extracell Vesicles. 2015 May 14;4:27066. doi: 10.3402/jev.v4.27066. eCollection 2015. J Extracell Vesicles. 2015. PMID: 25979354 Free PMC article.
References
-
- Howard AD, Kostura MJ, Thornberry N, Ding GJF, Limjuco G, Weidner J, et al. IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1beta precursor at two distinct sites and does not cleave 31-kDa IL-1alpha. J Immunol. 1991;147:2964–2969. - PubMed
-
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, et al. Molecular cloning of the interleukin-1Beta converting enzyme. Science. 1992;256:97–100. - PubMed
-
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1Beta processing in monocytes. Nature. 1992;356:768–774. - PubMed
-
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1B-converting enzyme. Cell. 1993;75:641–652. - PubMed
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87(2):171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

