Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin
- PMID: 19779615
- PMCID: PMC2745577
- DOI: 10.1371/journal.pone.0007152
Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin
Abstract
Background: Simple and effective vaccine administration is particularly important for annually recommended influenza vaccination. We hypothesized that vaccine delivery to the skin using a patch containing vaccine-coated microneedles could be an attractive approach to improve influenza vaccination compliance and efficacy.
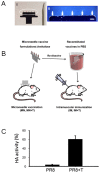
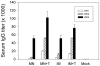
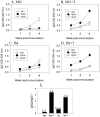
Methodology/principal findings: Solid microneedle arrays coated with inactivated influenza vaccine were prepared for simple vaccine delivery to the skin. However, the stability of the influenza vaccine, as measured by hemagglutination activity, was found to be significantly damaged during microneedle coating. The addition of trehalose to the microneedle coating formulation retained hemagglutination activity, indicating stabilization of the coated influenza vaccine. For both intramuscular and microneedle skin immunization, delivery of un-stabilized vaccine yielded weaker protective immune responses including viral neutralizing antibodies, protective efficacies, and recall immune responses to influenza virus. Immunization using un-stabilized vaccine also shifted the pattern of antibody isotypes compared to the stabilized vaccine. Importantly, a single microneedle-based vaccination using stabilized influenza vaccine was found to be superior to intramuscular immunization in controlling virus replication as well as in inducing rapid recall immune responses post challenge.
Conclusions/significance: The functional integrity of hemagglutinin is associated with inducing improved protective immunity against influenza. Simple microneedle influenza vaccination in the skin produced superior protection compared to conventional intramuscular immunization. This approach is likely to be applicable to other vaccines too.
Conflict of interest statement
Figures

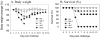

Similar articles
-
Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization.J Virol. 2010 Aug;84(15):7760-9. doi: 10.1128/JVI.01849-09. Epub 2010 May 19. J Virol. 2010. PMID: 20484519 Free PMC article.
-
Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch.Clin Vaccine Immunol. 2013 Sep;20(9):1433-9. doi: 10.1128/CVI.00251-13. Epub 2013 Jul 17. Clin Vaccine Immunol. 2013. PMID: 23863506 Free PMC article.
-
Microneedle-based vaccines.Curr Top Microbiol Immunol. 2009;333:369-93. doi: 10.1007/978-3-540-92165-3_18. Curr Top Microbiol Immunol. 2009. PMID: 19768415 Free PMC article. Review.
-
Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles.J Infect Dis. 2010 Jan 15;201(2):190-8. doi: 10.1086/649228. J Infect Dis. 2010. PMID: 20017632 Free PMC article.
-
Microneedle and mucosal delivery of influenza vaccines.Expert Rev Vaccines. 2012 May;11(5):547-60. doi: 10.1586/erv.12.25. Expert Rev Vaccines. 2012. PMID: 22697052 Free PMC article. Review.
Cited by
-
Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization.J Virol. 2010 Aug;84(15):7760-9. doi: 10.1128/JVI.01849-09. Epub 2010 May 19. J Virol. 2010. PMID: 20484519 Free PMC article.
-
Microneedles for drug and vaccine delivery.Adv Drug Deliv Rev. 2012 Nov;64(14):1547-68. doi: 10.1016/j.addr.2012.04.005. Epub 2012 May 1. Adv Drug Deliv Rev. 2012. PMID: 22575858 Free PMC article. Review.
-
Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles.J Control Release. 2013 Dec 10;172(2):579-88. doi: 10.1016/j.jconrel.2013.04.016. Epub 2013 Apr 30. J Control Release. 2013. PMID: 23643528 Free PMC article.
-
Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication.Nutrients. 2015 Feb 4;7(2):1021-36. doi: 10.3390/nu7021021. Nutrients. 2015. PMID: 25658239 Free PMC article.
-
Influenza virus-like particles containing M2 induce broadly cross protective immunity.PLoS One. 2011 Jan 18;6(1):e14538. doi: 10.1371/journal.pone.0014538. PLoS One. 2011. PMID: 21267073 Free PMC article.
References
-
- Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. - PubMed
-
- Compans RW, Klenk HD, Caliguiri LA, Choppin PW. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970;42:880–889. - PubMed
-
- Schulze IT. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970;42:890–904. - PubMed
-
- Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25:1361–1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

