Plasma pharmacokinetics and tissue disposition of novel dextran-methylprednisolone conjugates with peptide linkers in rats
- PMID: 19780131
- PMCID: PMC3415712
- DOI: 10.1002/jps.21934
Plasma pharmacokinetics and tissue disposition of novel dextran-methylprednisolone conjugates with peptide linkers in rats
Abstract
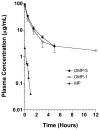
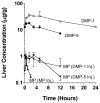
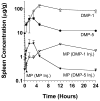
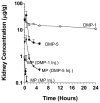
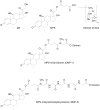
The plasma and tissue disposition of two novel dextran prodrugs of methylprednisolone (MP) containing one (DMP-1) or five (DMP-5) amino acids as linkers were studied in rats. Single 5-mg/kg doses (MP equivalent) of each prodrug or MP were administered intravenously, and blood and tissue samples were collected. Prodrug and drug concentrations were quantitated using HPLC, and noncompartmental pharmacokinetic parameters were estimated. Whereas conjugation of MP with dextran in both prodrugs substantially decreased the clearance of the drug by approximately 200-fold, the accumulations of the drug in the liver, spleen, and kidneys were significantly increased by conjugation. However, the extent of accumulation of DMP-1 in these tissues was substantially greater than that for DMP-5. Substantial amounts of MP were regenerated from both prodrugs in the liver and spleen, with the rate of release from DMP-5 being twice as fast as that from DMP-1. However, the AUCs of MP regenerated from DMP-1 in the liver and spleen were substantially higher than those after DMP-5. In contrast, in the kidneys, the AUC of MP regenerated from DMP-5 was higher than that after DMP-1 administration. These data suggest that DMP-1 may be more suitable than DMP-5 for targeting immunosuppression to the liver and spleen.
2009 Wiley-Liss, Inc. and the American Pharmacists Association
Figures
References
-
- Evans R, Manninen D, Dong F, Ascher N, Frist W, Hansen J, Kirklin J, Perkins J, Pirsch J, Sanfilippo F. Immunosuppressive therapy as a determinant of transplantation outcomes. Transplantation. 1993;55:1297–1305. - PubMed
-
- Wiesner R, Ludwig J, Krom R, Steers J, Porayko M, Gores G, Hay J. Treatment of early cellular rejection following liver transplantation with intravenous methylprednisolone. The effect of dose on response. Transplantation. 1994;58:1053–1056. - PubMed
-
- Volpin R, Angeli P, Galioto A, Fasolato S, Neri D, Barbazza F, Merenda R, Del Piccolo F, Strazzabosco M, Casagrande F, Feltracco P, Sticca A, Merkel C, Gerunda G, Gatta A. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8:527–534. - PubMed
-
- Millis JM. Treatment of liver allograft rejection. Liver Transpl Surg. 1999;5:S98–S106. - PubMed
-
- Adams D, Neuberger JM. Treatment of acute rejection. Semin Liver Dis. 1992;12:80–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

