The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease
- PMID: 19780638
- PMCID: PMC2787517
- DOI: 10.1080/10409230903193307
The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease
Abstract
A common feature of all eukaryotic membranes is the non-random distribution of different lipid species in the lipid bilayer (lipid asymmetry). Lipid asymmetry provides the two sides of the plasma membrane with different biophysical properties and influences numerous cellular functions. Alteration of lipid asymmetry plays a prominent role during cell fusion, activation of the coagulation cascade, and recognition and removal of apoptotic cell corpses by macrophages (programmed cell clearance). Here we discuss the origin and maintenance of phospholipid asymmetry, based on recent studies in mammalian systems as well as in Caenhorhabditis elegans and other model organisms, along with emerging evidence for a conserved role of mitochondria in the loss of lipid asymmetry during apoptosis. The functional significance of lipid asymmetry and its disruption during health and disease is also discussed.
Figures

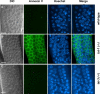
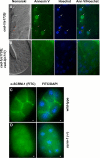
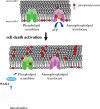
References
-
- Arroyo A, Modriansky M, Serinkan FB, Bello RI, Matsura T, Jiang J, Tyurin VA, Tyurina YY, Fadeel B, Kagan VE. NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO-differentiated HL-60 cells. Role in phagocytic clearance. J Biol Chem. 2002;277:49965–49975. - PubMed
-
- Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. - PubMed
-
- Aussel C, Pelassy C, Breittmayer JP. CD95 (Fas/APO-1) induces an increased phosphatidylserine synthesis that precedes its externalization during programmed cell death. FEBS Lett. 1998;431:195–199. - PubMed
-
- Bassé F, Stout JG, Sims PJ, Wiedmer T. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem. 1996;271:17205–17210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
