Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases
- PMID: 19781581
- PMCID: PMC2787696
- DOI: 10.1016/j.bbamcr.2009.09.013
Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases
Abstract
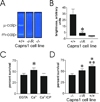
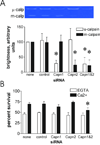
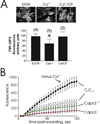
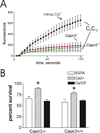
Mechanically damaged plasma membrane undergoes rapid calcium-dependent resealing that appears to depend, at least in part, on calpain-mediated cortical cytoskeletal remodeling. Cells null for Capns1, the non-catalytic small subunit present in both m- and mu-calpains, do not undergo calcium-mediated resealing. However, it is not known which of these calpains is needed for repair, or whether other major cytosolic proteinases may participate. Utilizing isozyme-selective siRNAs to decrease expression of Capn1 or Capn2, catalytic subunits of mu- and m-calpains, respectively, in a mouse embryonic fibroblast cell line, we now show that substantial loss of both activities is required to compromise calcium-mediated survival after cell scrape-damage. Using skeletal myotubes derived from Capn3-null mice, we were unable to demonstrate loss of sarcolemma resealing after needle scratch or laser damage. Isolated muscle fibers from Capn3 knockout mice also efficiently repaired laser damage. Employing either a cell line expressing a temperature sensitive E1 ubiquitin ligase, or lactacystin, a specific proteasome inhibitor, it was not possible to demonstrate an effect of the proteasome on calcium-mediated survival after injury. Moreover, several cell-permeant caspase inhibitors were incapable of significantly decreasing survival or inhibiting membrane repair. Taken together with previous studies, the results show that m- or mu-calpain can facilitate repair of damaged plasma membrane. While there was no evidence for the involvement of calpain-3, the proteasome or caspases in early events of plasma membrane repair, our studies do not rule out their participation in downstream events that may link plasma membrane repair to adaptive remodeling after injury.
Figures



References
-
- McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. - PubMed
-
- McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. - PubMed
-
- Fischer TA, McNeil PL, Khakee R, Finn P, Kelly RA, Pfeffer MA, Pfeffer JM. Cardiac myocyte membrane wounding in the abruptly pressure-overloaded rat heart under high wall stress. Hypertension. 1997;30:1041–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

