PPIB mutations cause severe osteogenesis imperfecta
- PMID: 19781681
- PMCID: PMC2756556
- DOI: 10.1016/j.ajhg.2009.09.001
PPIB mutations cause severe osteogenesis imperfecta
Abstract
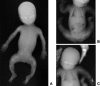
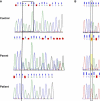
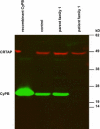
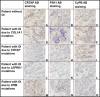
Deficiency of cartilage-associated protein (CRTAP) or prolyl 3-hydroxylase 1(P3H1) has been reported in autosomal-recessive lethal or severe osteogenesis imperfecta (OI). CRTAP, P3H1, and cyclophilin B (CyPB) form an intracellular collagen-modifying complex that 3-hydroxylates proline at position 986 (P986) in the alpha1 chains of collagen type I. This 3-prolyl hydroxylation is decreased in patients with CRTAP and P3H1 deficiency. It was suspected that mutations in the PPIB gene encoding CyPB would also cause OI with decreased collagen 3-prolyl hydroxylation. To our knowledge we present the first two families with recessive OI caused by PPIB gene mutations. The clinical phenotype is compatible with OI Sillence type II-B/III as seen with COL1A1/2, CRTAP, and LEPRE1 mutations. The percentage of 3-hydroxylated P986 residues in patients with PPIB mutations is decreased in comparison to normal, but it is higher than in patients with CRTAP and LEPRE1 mutations. This result and the fact that CyPB is demonstrable independent of CRTAP and P3H1, along with reported decreased 3-prolyl hydroxylation due to deficiency of CRTAP lacking the catalytic hydroxylation domain and the known function of CyPB as a cis-trans isomerase, suggest that recessive OI is caused by a dysfunctional P3H1/CRTAP/CyPB complex rather than by the lack of 3-prolyl hydroxylation of a single proline residue in the alpha1 chains of collagen type I.
Figures

References
-
- Marini J.C., Cabral W.A., Barnes A.M., Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007;6:1675–1681. - PubMed
-
- Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118:1341–1353. - PubMed
-
- Engel J., Prockop D.J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu. Rev. Biophys. Biophys. Chem. 1991;20:137–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

