Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation
- PMID: 19781996
- PMCID: PMC2785508
- DOI: 10.1016/j.molmed.2009.08.002
Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation
Abstract
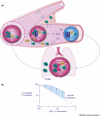
The protected transport of nitric oxide (NO) by hemoglobin (Hb) links the metabolic activity of working tissue to the regulation of its local blood supply through hypoxic vasodilation. This physiologic mechanism is allosterically coupled to the O(2) saturation of Hb and involves the covalent binding of NO to a cysteine residue in the beta-chain of Hb (Cys beta93) to form S-nitrosohemoglobin (SNO-Hb). Subsequent S-transnitrosation, the transfer of NO groups to thiols on the RBC membrane and then in the plasma, preserves NO vasodilator activity for delivery to the vascular endothelium. This SNO-Hb paradigm provides insight into the respiratory cycle and a new therapeutic focus for diseases involving abnormal microcirculatory perfusion. In addition, the formation of S-nitrosothiols in other proteins may regulate an array of physiological functions.
Figures
References
-
- Zelis R, Mason DT. Mechanism of systemic hemodynamic response during limb reactive hyperemia. Am J Physiol. 1969;217:1742–1746. - PubMed
-
- Crawford DG, et al. Oxygen lack as a possible cause of reactive hyperemia. Am J Physiol. 1959;197:613–616. - PubMed
-
- Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation. 2008;117:594–597. - PubMed
-
- Maher AR, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. - PubMed
-
- Hunter CJ, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

