Bone marrow engraftment but limited expansion of hematopoietic cells from multipotent germline stem cells derived from neonatal mouse testis
- PMID: 19782120
- PMCID: PMC2944399
- DOI: 10.1016/j.exphem.2009.09.006
Bone marrow engraftment but limited expansion of hematopoietic cells from multipotent germline stem cells derived from neonatal mouse testis
Abstract
Objective: Multipotent germline stem (mGS) cells derived from neonatal mouse testis, similar to embryonic stem (ES) cells, differentiate into various types of somatic cells in vitro and produce teratomas after inoculation into mice. In the present work, we examined mGS cells for hematopoietic progenitor potential in vitro and in vivo.
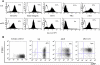
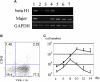
Materials and methods: mGS cells were differentiated on OP9 stromal cells and induced into Flk1(+) cells. Flk1(+) cells were sorted and replated on OP9 stromal cells with various cytokines and emerging hematopoietic cells were analyzed for lineage marker expression by fluorescein-activated cell sorting, progenitor activity by colony assay, and stem cell transplantation assay.
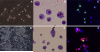
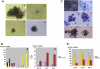
Results: mGS cells, like ES cells, produce hematopoietic progenitors, including both primitive and definitive erythromyeloid, megakaryocyte, and B- and T-cell lineages via Flk1(+) progenitors. When transplanted into the bone marrow (BM) of nonobese diabetic/severe combined immunodeficient (NOD/SCID) gammac(null) mice directly, mGS-derived green fluorescent protein (GFP)-positive cells were detected 4 months later in the BM and spleen. GFP(+) donor cells were also identified in the Hoechst33342 side population, a feature of hematopoietic stem cells. However, these mGS-derived hematopoietic cells did not proliferate in vivo, even after exposure to hematopoietic stressors, such as 5-fluorouracil (5FU) injection or serial transplantation.
Conclusion: mGS cells produced multipotent hematopoietic progenitor cells with myeloid and lymphoid lineage potential in vitro and localized in the BM after intra-BM injection but, like ES cells, failed to expand or show stem cell repopulating ability in vivo.
Figures




References
-
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. - PubMed
-
- Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. - PubMed
-
- Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. - PubMed
-
- Kataoka H, Takakura N, Nishikawa S, et al. Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ. 1997;39:729–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

