Myocardial perfusion, function, and dyssynchrony in patients with heart failure: baseline results from the single-photon emission computed tomography imaging ancillary study of the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Trial
- PMID: 19782789
- PMCID: PMC2908486
- DOI: 10.1016/j.ahj.2009.07.009
Myocardial perfusion, function, and dyssynchrony in patients with heart failure: baseline results from the single-photon emission computed tomography imaging ancillary study of the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Trial
Abstract
Background: There are currently limited data on the relationships between resting perfusion abnormalities, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) functional class, and exercise capacity as defined by peak VO(2) and 6-minute walk test in patients with heart failure (HF) and reduced LVEF. Furthermore, the association between resting perfusion abnormalities and left ventricular dyssynchrony is currently unknown. This article addresses the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) gated SPECT imaging (gSPECT) substudy baseline results.
Methods: HF-ACTION was a multicenter, randomized controlled trial of aerobic exercise training versus usual care in 2,331 stable patients with LVEF of < or = 35% and NYHA class II to IV HF symptoms treated with optimal medical therapy. Subjects enrolled in the HF-ACTION substudy underwent resting Tc-99m tetrofosmin gSPECT at baseline (n = 240). Images were evaluated for extent and severity of perfusion abnormalities using a 17-segment and a 5-degree gradation severity score (summed rest score [SRS]). Left ventricular function and dyssynchrony were assessed using validated available commercial software.
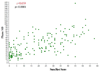
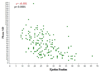
Results: The average age of patients enrolled was 59, 69% were male, 63% were white, and 33% were African American. Of the 240 participants, 129 (54%) were ischemic and 111 (46%) were nonischemic in etiology. The median LVEF by gSPECT for the entire cohort was 26%. Among the nuclear variables, there was a modest correlation between LVEF and SRS (r = -0.31, P < .0001) and there were stronger correlations between phase SD and SRS (r = 0.66, P < .0001) as well as phase SD and LVEF (r = -0.50, P < .0001). Patients with NYHA class III symptoms had more severe and significant degrees of dyssynchrony (median phase SD 54 degrees ) than those with NYHA class II symptoms (median phase SD 39 degrees, P = .001). Patients with an ischemic etiology had a higher SRS (P < .0001) and significantly more dyssynchrony (P < .0001) than those who were nonischemic. However, there was no difference in LVEF or objective measures of exercise capacity between these groups. With respect to peak VO(2), there was a weak correlation with LVEF (r = 0.18, P = .006) and no correlation with SRS (r = -0.04, P = 0.59) or with dyssynchrony (r = -0.13, P = .09). A weak but statistically significant correlation between SRS and 6-minute walk was observed (r = -0.15, P = .047).
Conclusions: Gated SPECT imaging can provide important information in patients with HF due to severe LV dysfunction including quantitative measures of global systolic function, perfusion, and dyssynchrony. These measurements are modestly but significantly related to symptom severity and objective measures of exercise capacity.
Trial registration: ClinicalTrials.gov NCT00047437.
Conflict of interest statement
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. Dr. Atchley is a fellow in training and is funded by a National Institutes of Health T32 research grant. Dr. Borges-Neto is part of the speakers’ bureau and advisory board and receives grants from GE Health. Dr. Ellis receives funding support from GE Health. Drs. Kraus, Iskandrian, Whellan, and Kitzman have no conflicts of interest to disclose.
Figures




References
-
- Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–e82. - PubMed
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. - PubMed
-
- Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. - PubMed
-
- Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–2694. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL064257/HL/NHLBI NIH HHS/United States
- 5U01HL066461/HL/NHLBI NIH HHS/United States
- 5U01HL064264/HL/NHLBI NIH HHS/United States
- U01 HL064250/HL/NHLBI NIH HHS/United States
- P60AG10484/AG/NIA NIH HHS/United States
- U01 HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066497/HL/NHLBI NIH HHS/United States
- U01 HL064264/HL/NHLBI NIH HHS/United States
- T32 HL069749/HL/NHLBI NIH HHS/United States
- 5U01HL066482/HL/NHLBI NIH HHS/United States
- 5U01HL066497/HL/NHLBI NIH HHS/United States
- U01 HL068980/HL/NHLBI NIH HHS/United States
- 5U01HL064250/HL/NHLBI NIH HHS/United States
- 5U01HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066491/HL/NHLBI NIH HHS/United States
- 5U01HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL064257/HL/NHLBI NIH HHS/United States
- U01 HL066482/HL/NHLBI NIH HHS/United States
- 5U01HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066491/HL/NHLBI NIH HHS/United States
- R37AG18915/AG/NIA NIH HHS/United States
- R37 AG018915/AG/NIA NIH HHS/United States
- U01 HL066494/HL/NHLBI NIH HHS/United States
- 5U01HL066501/HL/NHLBI NIH HHS/United States
- U01 HL066461/HL/NHLBI NIH HHS/United States
- U01 HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL068980/HL/NHLBI NIH HHS/United States
- U01 HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066494/HL/NHLBI NIH HHS/United States
- U01 HL066501/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

