Motoneuron excitability: the importance of neuromodulatory inputs
- PMID: 19783207
- PMCID: PMC7312725
- DOI: 10.1016/j.clinph.2009.08.009
Motoneuron excitability: the importance of neuromodulatory inputs
Abstract
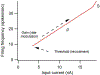
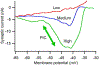
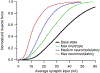
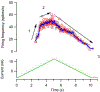
The excitability of spinal motoneurons is both fundamental for motor behavior and essential in diagnosis of neural disorders. There are two mechanisms for altering this excitability. The classic mechanism is mediated by synaptic inputs that depolarize or hyperpolarize motoneurons by generating postsynaptic potentials. This "ionotropic" mechanism works via neurotransmitters that open ion channels in the cell membrane. In the second mechanism, neurotransmitters bind to receptors that activate intracellular signaling pathways. These pathways modulate the properties of the voltage-sensitive channels that determine the intrinsic input-output properties of motoneurons. This "neuromodulatory" mechanism usually does not directly activate motoneurons but instead dramatically alters the neuron's response to ionotropic inputs. We present extensive evidence that neuromodulatory inputs exert a much more powerful effect on motoneuron excitability than ionotropic inputs. The most potent neuromodulators are probably serotonin and norepinephrine, which are released by axons originating in the brainstem and can increase motoneuron excitability fivefold or more. Thus, the standard tests of motoneuron excitability (H-reflexes, tendon taps, tendon vibration and stretch reflexes) are strongly influenced by the level of neuromodulatory input to motoneurons. This insight is likely to be profoundly important for clinical diagnosis and treatment.
Figures




References
-
- Alaburda A, Perrier JF, Hounsgaard J (2002) Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol 508:219–226. - PubMed
-
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE (1998) Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha- motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393:69–83. - PubMed
-
- Amendola J, Durand J (2008) Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol 511:329–341. - PubMed
-
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

