Preventing Ataxin-3 protein cleavage mitigates degeneration in a Drosophila model of SCA3
- PMID: 19783548
- PMCID: PMC2778376
- DOI: 10.1093/hmg/ddp456
Preventing Ataxin-3 protein cleavage mitigates degeneration in a Drosophila model of SCA3
Abstract
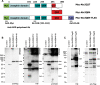
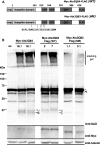
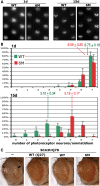
Protein cleavage is a common feature in human neurodegenerative disease. Ataxin-3 protein with an expanded polyglutamine (polyQ) repeat causes spinocerebellar ataxia type-3 (SCA3), also called Machado-Joseph disease, and is cleaved in mammalian cells, transgenic mice and SCA3 patient brain tissue. However, the pathological significance of Ataxin-3 cleavage has not been carefully examined. To gain insight into the significance of Ataxin-3 cleavage, we developed a Drosophila SL2 cell-based model as well as transgenic fly models. Our data indicate that Ataxin-3 protein cleavage is conserved in the fly and may be caspase-dependent as reported previously. Importantly, comparison of flies expressing either wild-type or caspase-site mutant proteins indicates that Ataxin-3 cleavage enhances neuronal loss in vivo. This genetic in vivo confirmation of the pathological role of Ataxin-3 cleavage indicates that therapies targeting Ataxin-3 cleavage might slow disease progression in SCA3 patients.
Figures


References
-
- Beher D. Gamma-secretase modulation and its promise for Alzheimer's disease: a rationale for drug discovery. Curr. Top. Med. Chem. 2008;8:34–37. - PubMed
-
- Newman J., Rissman R.A., Sarsoza F., Kim R.C., Dick M., Bennett D.A., Cotman C.W., Rohn T.T., Head E. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and alpha-synuclein pathology. Acta Neuropathol. 2005;110:135–144. - PubMed
-
- Hoffner G., Island M.L., Djian P. Purification of neuronal inclusions of patients with Huntington's disease reveals a broad range of N-terminal fragments of expanded huntingtin and insoluble polymers. J. Neurochem. 2005;95:125–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

