Epigenetic regulation of gene programs by EMF1 and EMF2 in Arabidopsis
- PMID: 19783648
- PMCID: PMC2815887
- DOI: 10.1104/pp.109.143495
Epigenetic regulation of gene programs by EMF1 and EMF2 in Arabidopsis
Abstract
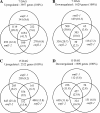
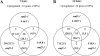
The EMBRYONIC FLOWER (EMF) genes are required to maintain vegetative development in Arabidopsis (Arabidopsis thaliana). Loss-of-function emf mutants skip the vegetative phase, flower upon germination, and display pleiotropic phenotypes. EMF1 encodes a putative transcriptional regulator, while EMF2 encodes a Polycomb group (PcG) protein. PcG proteins form protein complexes that maintain gene silencing via histone modification. They are known to function as master regulators repressing multiple gene programs. Both EMF1 and EMF2 participate in PcG-mediated silencing of the flower homeotic genes AGAMOUS, PISTILLATA, and APETALA3. Full-genome expression pattern analysis of emf mutants showed that both EMF proteins regulate additional gene programs, including photosynthesis, seed development, hormone, stress, and cold signaling. Chromatin immunoprecipitation was carried out to investigate whether EMF regulates these genes directly. It was determined that EMF1 and EMF2 interact with genes encoding the transcription factors ABSCISIC ACID INSENSITIVE3, LONG VEGETATIVE PHASE1, and FLOWERING LOCUS C, which control seed development, stress and cold signaling, and flowering, respectively. Our results suggest that the two EMFs repress the regulatory genes of individual gene programs to effectively silence the genetic pathways necessary for vegetative development and stress response. A model of the regulatory network mediated by EMF is proposed.
Figures





Similar articles
-
EMF genes maintain vegetative development by repressing the flower program in Arabidopsis.Plant Cell. 2003 Mar;15(3):681-93. doi: 10.1105/tpc.007831. Plant Cell. 2003. PMID: 12615941 Free PMC article.
-
EMBRYONIC FLOWER1 participates in polycomb group-mediated AG gene silencing in Arabidopsis.Plant Cell. 2008 Feb;20(2):277-91. doi: 10.1105/tpc.106.049957. Epub 2008 Feb 15. Plant Cell. 2008. PMID: 18281509 Free PMC article.
-
Temporal and spatial requirement of EMF1 activity for Arabidopsis vegetative and reproductive development.Mol Plant. 2009 Jul;2(4):643-653. doi: 10.1093/mp/ssp004. Epub 2009 Mar 24. Mol Plant. 2009. PMID: 19825645
-
Mechanisms of floral repression in Arabidopsis.Curr Opin Plant Biol. 2003 Feb;6(1):29-35. doi: 10.1016/s1369-5266(02)00014-6. Curr Opin Plant Biol. 2003. PMID: 12495748 Review.
-
Polycomb group and trithorax group proteins in Arabidopsis.Biochim Biophys Acta. 2007 May-Jun;1769(5-6):375-82. doi: 10.1016/j.bbaexp.2007.01.010. Epub 2007 Feb 7. Biochim Biophys Acta. 2007. PMID: 17363079 Review.
Cited by
-
Genome-wide association mapping of flowering time in Arabidopsis thaliana in nature: genetics for underlying components and reaction norms across two successive years.Acta Bot Gall. 2013 Jul 1;160(3-4):205-219. doi: 10.1080/12538078.2013.807302. Acta Bot Gall. 2013. PMID: 24470785 Free PMC article.
-
Transcription factor VRT2 reinitiates vernalization when interrupted by warm temperatures in a temperate grass model.Plant Physiol. 2024 Dec 2;196(4):2614-2624. doi: 10.1093/plphys/kiae498. Plant Physiol. 2024. PMID: 39316702 Free PMC article.
-
Knowing When to Silence: Roles of Polycomb-Group Proteins in SAM Maintenance, Root Development, and Developmental Phase Transition.Int J Mol Sci. 2020 Aug 15;21(16):5871. doi: 10.3390/ijms21165871. Int J Mol Sci. 2020. PMID: 32824274 Free PMC article. Review.
-
EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development.PLoS Genet. 2012;8(3):e1002512. doi: 10.1371/journal.pgen.1002512. Epub 2012 Mar 22. PLoS Genet. 2012. PMID: 22457632 Free PMC article.
-
Dynamics of H3K27me3 Modification on Plant Adaptation to Environmental Cues.Plants (Basel). 2021 Jun 8;10(6):1165. doi: 10.3390/plants10061165. Plants (Basel). 2021. PMID: 34201297 Free PMC article. Review.
References
-
- Bai S, Sung ZR (1995) The role of EMF1 in regulating the vegetative and reproductive transition in Arabidopsis thaliana. Am J Bot 82 1095–1103
-
- Bowler C, Benvenuto G, Laflamme P, Molino D, Probst AV, Tariq M, Paszkowski J (2004) Chromatin techniques for plant cells. Plant J 39 776–789 - PubMed
-
- Calonje M, Sung ZR (2006) Complexity beneath the silence. Curr Opin Plant Biol 9 530–537 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

