GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase
- PMID: 19783659
- PMCID: PMC2781691
- DOI: 10.1074/jbc.M109.047910
GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase
Abstract
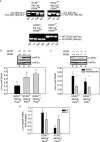
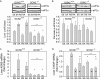
Asparaginase depletes circulating asparagine and glutamine, activating amino acid deprivation responses (AADR) such as phosphorylation of eukaryotic initiation factor 2 (p-eIF2) leading to increased mRNA levels of asparagine synthetase and CCAAT/enhancer-binding protein beta homologous protein (CHOP) and decreased mammalian target of rapamycin complex 1 (mTORC1) signaling. The objectives of this study were to assess the role of the eIF2 kinases and protein kinase R-like endoplasmic reticulum resident kinase (PERK) in controlling AADR to asparaginase and to compare the effects of asparaginase on mTORC1 to that of rapamycin. In experiment 1, asparaginase increased hepatic p-eIF2 in wild-type mice and mice with a liver-specific PERK deletion but not in GCN2 null mice nor in GCN2-PERK double null livers. In experiment 2, wild-type and GCN2 null mice were treated with asparaginase (3 IU per g of body weight), rapamycin (2 mg per kg of body weight), or both. In wild-type mice, asparaginase but not rapamycin increased p-eIF2, p-ERK1/2, p-Akt, and mRNA levels of asparagine synthetase and CHOP in liver. Asparaginase and rapamycin each inhibited mTORC1 signaling in liver and pancreas but maximally together. In GCN2 null livers, all responses to asparaginase were precluded except CHOP mRNA expression, which remained partially elevated. Interestingly, rapamycin blocked CHOP induction by asparaginase in both wild-type and GCN2 null livers. These results indicate that GCN2 is required for activation of AADR to asparaginase in liver. Rapamycin modifies the hepatic AADR to asparaginase by preventing CHOP induction while maximizing inhibition of mTORC1.
Figures

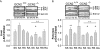
References
-
- Hill J. M., Roberts J., Loeb E., Khan A., MacLellan A., Hill R. W. (1967) JAMA 202, 882–888 - PubMed
-
- Holcenberg J. (2004) J. Pediatr. Hematol. Oncol. 26, 273–274 - PubMed
-
- Reinert R. B., Oberle L. M., Wek S. A., Bunpo P., Wang X. P., Mileva I., Goodwin L. O., Aldrich C. J., Durden D. L., McNurlan M. A., Wek R. C., Anthony T. G. (2006) J. Biol. Chem. 281, 31222–31233 - PubMed
-
- Bunpo P., Murray B., Cundiff J., Brizius E., Aldrich C. J., Anthony T. G. (2008) J. Nutr. 138, 338–343 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

