ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: evidence for heparan sulfate epitope specificity and role of both syndecan and glypican
- PMID: 19783663
- PMCID: PMC2781711
- DOI: 10.1074/jbc.M109.036129
ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: evidence for heparan sulfate epitope specificity and role of both syndecan and glypican
Abstract
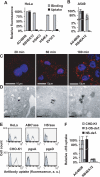
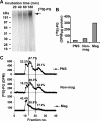
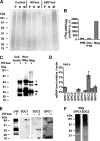
Cellular uptake of several viruses and polybasic macromolecules requires the expression of cell-surface heparan sulfate proteoglycan (HSPG) through as yet ill defined mechanisms. We unexpectedly found that among several cell-surface-binding single chain variable fragment (scFv) anti-HS antibody (alphaHS) clones, only one, AO4B08, efficiently translocated macromolecular cargo to intracellular vesicles through induction of HSPG endocytosis. Interestingly, AO4B08-induced PG internalization was strictly dependent on HS 2-O-sulfation and appeared independent of intact N-sulfation. AO4B08 and human immunodeficiency virus (HIV)-Tat, i.e. a well known cell-penetrating peptide, were shown to compete for the internalizing PG population. To obtain a more detailed characterization of this pathway, we have developed a procedure for the isolation of endocytic vesicles by conjugating AO4B08 with superparamagnetic nanoparticles. [(35)S]sulfate-labeled HSPG was found to accumulate in isolated, AO4B08-containing vesicles, providing the first biochemical evidence for intact HSPG co-internalization with its ligand. Further analysis revealed the existence of both syndecan, i.e. a transmembrane HSPG, and glycosyl-phosphatidyl-inositol-anchored glypican in purified vesicles. Importantly, internalized syndecan and glypican were found to co-localize in AO4B08-containing vesicles. Our data establish HSPGs as true internalizing receptors of macromolecular cargo and indicate that the sorting of cell-surface HSPG to endocytic vesicles is determined by a specific HS epitope that can be carried by both syndecan and glypican core protein.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

