Sonic hedgehog-dependent synthesis of laminin alpha1 controls basement membrane assembly in the myotome
- PMID: 19783738
- PMCID: PMC2752398
- DOI: 10.1242/dev.036087
Sonic hedgehog-dependent synthesis of laminin alpha1 controls basement membrane assembly in the myotome
Abstract
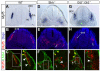
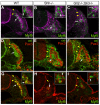
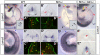
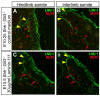
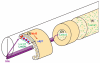
Basement membranes have essential structural and signalling roles in tissue morphogenesis during embryonic development, but the mechanisms that control their formation are still poorly understood. Laminins are key components of basement membranes and are thought to be essential for initiation of basement membrane assembly. Here, we report that muscle progenitor cells populating the myotome migrate aberrantly in the ventral somite in the absence of sonic hedgehog (Shh) signalling, and we show that this defect is due to the failure to form a myotomal basement membrane. We reveal that expression of Lama1, which encodes laminin alpha1, a subunit of laminin-111, is not activated in Shh(-/-) embryos. Recovery of Lama1 expression or addition of exogenous laminin-111 to Shh(-/-);Gli3(-/-) embryos restores the myotomal basement membrane, demonstrating that laminin-111 is necessary and sufficient to initiate assembly of the myotomal basement membrane. This study uncovers an essential role for Shh signalling in the control of laminin-111 synthesis and in the initiation of basement membrane assembly in the myotome. Furthermore, our data indicate that laminin-111 function cannot be compensated by laminin-511.
Figures


References
-
- Andac, Z., Sasaki, T., Mann, K., Brancaccio, A., Deutzmann, R. and Timpl, R. (1999). Analysis of heparin, alpha-dystroglycan and sulfatide binding to the G domain of the laminin alpha1 chain by site-directed mutagenesis. J. Mol. Biol. 287, 253-264. - PubMed
-
- Anderson, C., Winder, S. J. and Borycki, A. G. (2007). Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev. Dyn. 236, 2627-2635. - PubMed
-
- Bajanca, F., Luz, M., Duxson, M. J. and Thorsteinsdottir, S. (2004). Integrins in the mouse myotome: developmental changes and differences between the epaxial and hypaxial lineage. Dev. Dyn. 231, 402-415. - PubMed
-
- Bajanca, F., Luz, M., Raymond, K., Martins, G. G., Sonnenberg, A., Tajbakhsh, S., Buckingham, M. and Thorsteinsdottir, S. (2006). Integrin alpha6beta1-laminin interactions regulate early myotome formation in the mouse embryo. Development 133, 1635-1644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

