A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome
- PMID: 19783846
- PMCID: PMC2778371
- DOI: 10.1093/hmg/ddp438
A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome
Abstract
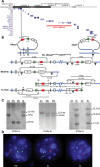
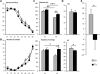
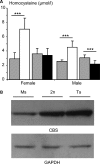
Mental retardation in Down syndrome (DS), the most frequent trisomy in humans, varies from moderate to severe. Several studies both in human and based on mouse models identified some regions of human chromosome 21 (Hsa21) as linked to cognitive deficits. However, other intervals such as the telomeric region of Hsa21 may contribute to the DS phenotype but their role has not yet been investigated in detail. Here we show that the trisomy of the 12 genes, found in the 0.59 Mb (Abcg1-U2af1) Hsa21 sub-telomeric region, in mice (Ts1Yah) produced defects in novel object recognition, open-field and Y-maze tests, similar to other DS models, but induces an improvement of the hippocampal-dependent spatial memory in the Morris water maze along with enhanced and longer lasting long-term potentiation in vivo in the hippocampus. Overall, we demonstrate the contribution of the Abcg1-U2af1 genetic region to cognitive defect in working and short-term recognition memory in DS models. Increase in copy number of the Abcg1-U2af1 interval leads to an unexpected gain of cognitive function in spatial learning. Expression analysis pinpoints several genes, such as Ndufv3, Wdr4, Pknox1 and Cbs, as candidates whose overexpression in the hippocampus might facilitate learning and memory in Ts1Yah mice. Our work unravels the complexity of combinatorial genetic code modulating different aspect of mental retardation in DS patients. It establishes definitely the contribution of the Abcg1-U2af1 orthologous region to the DS etiology and suggests new modulatory pathways for learning and memory.
Figures



References
-
- Antonarakis S.E., Lyle R., Dermitzakis E.T., Reymond A., Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat. Rev. Genet. 2004;5:725–738. - PubMed
-
- Nadel L. Down's syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–166. - PubMed
-
- Antonarakis S.E., Epstein C.J. The challenge of Down syndrome. Trends Mol. Med. 2006;12:473–479. - PubMed
-
- Gibson D., Groeneweg G., Jerry P., Harris A. Age and pattern of intellectual decline among Down syndrome and other mentally retarded adults. Int. J. Rehabil. Res. 1988;11:47–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

