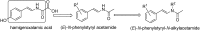Synthesis and anti-human immunodeficiency virus type 1 activity of (E)-N-phenylstyryl-N-alkylacetamide derivatives
- PMID: 19783916
- PMCID: PMC6254728
- DOI: 10.3390/molecules14093176
Synthesis and anti-human immunodeficiency virus type 1 activity of (E)-N-phenylstyryl-N-alkylacetamide derivatives
Abstract
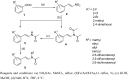
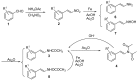
A series of (E)-N-phenylstyryl-N-alkylacetamides, 5, were synthesized by direct reduction-acetylation of beta-arylnitroolefins, followed by N-alkylation. The title compounds were characterized by (1)H-NMR, EIMS and IR analysis. All the synthesized compounds were assayed as HIV-1 non-nucleoside reverse transcriptase inhibitors. A SAR study revealed that when group R(1) in 5 was ortho-substituted, the resulting compounds showed better inhibitory activities against HIV-1 RT. Among the tested compounds, 5i (R(1) = 2-Br, R(2) = 3,5-difluorobenzyl) exhibited the highest enzyme activity, with a 88.89% inhibitory ratio against HIV-1 reverse transcriptase at the tested concentration. Further cell-based anti-HIV-1 assays showed that compound 5i exhibited a SI value of 29 with an EC(50) value of 4 microM in C8166 cells.
Figures
Similar articles
-
Synthesis and anti-HIV activity evaluation of 2-(4-(naphthalen-2-yl)-1,2,3-thiadiazol-5-ylthio)-N-acetamides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors.Eur J Med Chem. 2009 Nov;44(11):4648-53. doi: 10.1016/j.ejmech.2009.06.037. Epub 2009 Jul 4. Eur J Med Chem. 2009. PMID: 19628308
-
Novel 1,2,3-thiadiazole derivatives as HIV-1 NNRTIs with improved potency: Synthesis and preliminary SAR studies.Bioorg Med Chem. 2009 Aug 15;17(16):5920-7. doi: 10.1016/j.bmc.2009.07.004. Epub 2009 Jul 7. Bioorg Med Chem. 2009. PMID: 19620009
-
Arylazolylthioacetanilide. Part 8: Design, synthesis and biological evaluation of novel 2-(2-(2,4-dichlorophenyl)-2H-1,2,4-triazol-3-ylthio)-N-arylacetamides as potent HIV-1 inhibitors.Eur J Med Chem. 2011 Oct;46(10):5039-45. doi: 10.1016/j.ejmech.2011.08.011. Epub 2011 Aug 12. Eur J Med Chem. 2011. PMID: 21872971
-
Design, synthesis and biological evaluation of novel acetamide-substituted doravirine and its prodrugs as potent HIV-1 NNRTIs.Bioorg Med Chem. 2019 Feb 1;27(3):447-456. doi: 10.1016/j.bmc.2018.12.039. Epub 2018 Dec 30. Bioorg Med Chem. 2019. PMID: 30606670 Review.
-
Progress of bis(heteroaryl)piperazines (BHAPs) as non-nucleoside reverse transcriptase inhibitors (NNRTIs) against human immunodeficiency virus type 1 (HIV-1).Mini Rev Med Chem. 2010 Jan;10(1):62-72. doi: 10.2174/138955710791112578. Mini Rev Med Chem. 2010. PMID: 20380641 Review.
References
-
- Didierjean J., Isel C., Querré F., Mouscadet J.F., Aubertin A.M., Valnot J.Y., Piettre S.R., Marquet R. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, RNase H, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother. 2005;49:4884–4894. doi: 10.1128/AAC.49.12.4884-4894.2005. - DOI - PMC - PubMed
-
- Jones L.H., Allan G., Barba O., Burt C., Corbau R., Dupont T., Knochel T., Irving S., Middleton D.S., Mowbray C.E., Perros M., Ringrose H., Swain N.A., Webster R. Westby., M. Phillips Novel indazole non-nucleoside reverse transcriptase inhibitors using molecular hybridization based on crystallographic overlay. J. Med. Chem. 2009;52:1219–1223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous