Development of a high-throughput assay for screening of gamma-secretase inhibitor with endogenous human, mouse or Drosophila gamma-secretase
- PMID: 19783945
- PMCID: PMC6254802
- DOI: 10.3390/molecules14093589
Development of a high-throughput assay for screening of gamma-secretase inhibitor with endogenous human, mouse or Drosophila gamma-secretase
Abstract
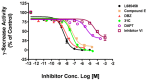
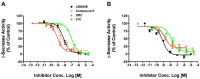
Selective lowering of amyloid-beta levels with small-molecule gamma-secretase inhibitors is a promising therapeutic approach for Alzheimer's disease. In this work, we developed a high throughput assay for screening of gamma-secretase inhibitors with endogenous gamma-secretase and a fluorogenic substrate. The IC(50) values of known gamma-secretase inhibitors generated with this method were comparable with reported values obtained by other methods. The assay was optimized and applied to a small-scale screening of 1,280 compounds. The discovery of several new inhibitors warrants further investigation. This assay was also proven to be easily adopted to test compounds for drosophila and mouse gamma-secretase, which could be very useful to assess compounds activity against gamma-secretase from different species before the in vivo test in animal models.
Figures



References
-
- Dovey H.F., John V., Anderson J.P., Chen L.Z., De S.A.P., Fang L.Y., Freedman S.B., Folmer B., Goldbach E., Holsztynska E.J., Hu K.L., Johnson-Wood K.L., Kennedy S.L., Kholodenko D., Knops J.E., Latimer L.H., Lee M., Liao Z., Lieberburg I.M., Motter R.N., Mutter L.C., Nietz J., Quinn K.P., Sacchi K.L., Seubert P.A., Shopp G.M., Thorsett E.D., Tung J.S., Wu J., Yang S., Yin C.T., Schenk D.B., May P.C., Altstiel L.D., Bender M.H., Boggs L.N., Britton T.C., Clemens J.C., Czilli D.L., Dieckman-McGinty D.K., Droste J.J., Fuson K.S., Gitter B.D., Hyslop P.A., Johnstone E.M., Li W.Y., Little S.P., Mabry T.E., Miller F.D., Audia J.E. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 2001:173–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

