Nasal delivery of high molecular weight drugs
- PMID: 19783956
- PMCID: PMC6254717
- DOI: 10.3390/molecules14093754
Nasal delivery of high molecular weight drugs
Abstract
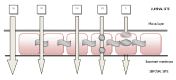
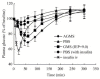
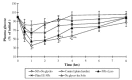
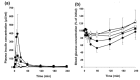
Nasal drug delivery may be used for either local or systemic effects. Low molecular weight drugs with are rapidly absorbed through nasal mucosa. The main reasons for this are the high permeability, fairly wide absorption area, porous and thin endothelial basement membrane of the nasal epithelium. Despite the many advantages of the nasal route, limitations such as the high molecular weight (HMW) of drugs may impede drug absorption through the nasal mucosa. Recent studies have focused particularly on the nasal application of HMW therapeutic agents such as peptide-protein drugs and vaccines intended for systemic effects. Due to their hydrophilic structure, the nasal bioavailability of peptide and protein drugs is normally less than 1%. Besides their weak mucosal membrane permeability and enzymatic degradation in nasal mucosa, these drugs are rapidly cleared from the nasal cavity after administration because of mucociliary clearance. There are many approaches for increasing the residence time of drug formulations in the nasal cavity resulting in enhanced drug absorption. In this review article, nasal route and transport mechanisms across the nasal mucosa will be briefly presented. In the second part, current studies regarding the nasal application of macromolecular drugs and vaccines with nano- and micro-particulate carrier systems will be summarised.
Figures
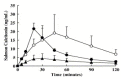
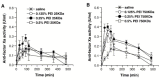

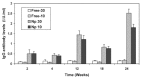
References
-
- Cornaz A.L., Buri P. Nasal Mucosa as an Absorption Barrier. Eur. J. Pharm. Biopharm. 1994;40:261–270.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

