CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling
- PMID: 19785013
- PMCID: PMC3385349
- DOI: 10.1002/stem.222
CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling
Abstract
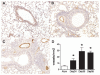
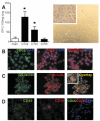
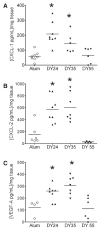
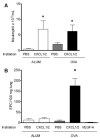
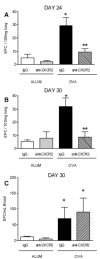
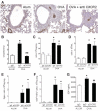
Airway remodeling is a central feature of asthma and includes the formation of new peribronchial blood vessels, which is termed angiogenesis. In a number of disease models, bone marrow-derived endothelial progenitor cells (EPCs) have been shown to contribute to the angiogenic response. In this study we set out to determine whether EPCs were recruited into the lungs in a model of allergic airways disease and to identify the factors regulating EPC trafficking in this model. We observed a significant increase in the number of peribronchial blood vessels at day 24, during the acute inflammatory phase of the model. This angiogenic response was associated with an increase in the quantity of EPCs recoverable from the lung. These EPCs formed colonies after 21 days in culture and were shown to express CD31, von Willebrand factor, and vascular endothelial growth factor (VEGF) receptor 2, but were negative for CD45 and CD14. The influx in EPCs was associated with a significant increase in the proangiogenic factors VEGF-A and the CXCR2 ligands, CXCL1 and CXCL2. However, we show directly that, while the CXCL1 and CXCL2 chemokines can recruit EPCs into the lungs of allergen-sensitized mice, VEGF-A was ineffective in this respect. Further, the blockade of CXCR2 significantly reduced EPC numbers in the lungs after allergen exposure and led to a decrease in the numbers of peribronchial blood vessels after allergen challenge with no effect on inflammation. The data presented here provide in vivo evidence that CXCR2 is critical for both EPC recruitment and the angiogenic response in this model of allergic inflammation of the airways.
Figures
References
-
- Zisch AH. Tissue engineering of angiogenesis with autologous endothelial progenitor cells. Curr Opin Biotechnol. 2004;15:424–429. - PubMed
-
- Asosingh K, Swaidani S, Aronica M, et al. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–6494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

