PEGylated dendritic unimolecular micelles as versatile carriers for ligands of G protein-coupled receptors
- PMID: 19785401
- PMCID: PMC2891302
- DOI: 10.1021/bc9001689
PEGylated dendritic unimolecular micelles as versatile carriers for ligands of G protein-coupled receptors
Abstract
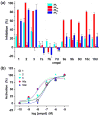
Despite its widespread application in nanomedicine, poly(ethylene glycol) (PEG) is seldom used for covalent modification of ligands for G protein-coupled receptors (GPCRs) due to potential steric complications. In order to study the influence of PEG chains on the biological activity of GPCR ligands bound to a common macromolecular carrier, we prepared a series of G3 polyamidoamine (PAMAM) dendrimers derivatized with Alexa Fluor 488, varying numbers of PEG(550)/PEG(750)/PEG(2000), and nucleoside moieties derived from the A(2A) adenosine receptor (AR) agonist CGS21680 (2-[4-(2-carboxylethyl)phenylethylamino]-5'-N-ethylcarboxamidoadenosine). These dendrimer conjugates were purified by size exclusion chromatography and characterized by (1)H NMR and MALDI MS. In radioligand binding assays, some PAMAM-PEG conjugates showed enhanced subtype-selectivity at the human A(2A) AR compared to monomeric ligands of comparable affinity. The functional potency was measured in the A(2A) AR-mediated activation of adenylate cyclase and inhibition of ADP-induced platelet aggregation. Interestingly, the dendrimer conjugate 10c bearing 11 PEG(750) chains (out of theoretical 32 amino end groups) and 14 nucleoside moieties was 5-fold more potent in A(2A) AR-mediated stimulation of cyclic AMP formation than 10d with 4 PEG(2000) chains and 21 nucleosides, although the binding affinities of these 2 compounds were similar. Thus, a relatively small (≤10 nm) multivalent ligand 10c modified for water solubility maintained high potency and displayed increased A(2A) AR binding selectivity over the monomeric nucleosides. The current study demonstrates the feasibility of using short PEG chains in the design of carriers that target ligand-receptor interactions.
Figures




References
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007;2:751–760. - PubMed
-
- Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nature Rev Drug Discov. 2003;2:347–360. - PubMed
-
- Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–67. - PubMed
-
- Boas U, Christensen JB, Heegaard PMH. Dendrimers in medicine and biotechnology. The Royal Society of Chemistry; Cambridge: 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

