Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties
- PMID: 19785662
- PMCID: PMC2785536
- DOI: 10.1111/j.1476-5381.2009.00383.x
Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties
Abstract
Background and purpose: ABC multidrug transporters (MDR-ABC proteins) cause multiple drug resistance in cancer and may be involved in the decreased anti-cancer efficiency and modified pharmacological properties of novel specifically targeted agents. It has been documented that ABCB1 and ABCG2 interact with several first-generation, small-molecule, tyrosine kinase inhibitors (TKIs), including the Bcr-Abl fusion kinase inhibitor imatinib, used for the treatment of chronic myeloid leukaemia. Here, we have investigated the specific interaction of these transporters with nilotinib, dasatinib and bosutinib, three clinically used, second-generation inhibitors of the Bcr-Abl tyrosine kinase activity.
Experimental approach: MDR-ABC transporter function was screened in both membrane- and cell-based (K562 cells) systems. Cytotoxicity measurements in Bcr-Abl-positive model cells were coupled with direct determination of intracellular TKI concentrations by high-pressure liquid chromatography-mass spectrometry and analysis of the pattern of Bcr-Abl phosphorylation. Transporter function in membranes was assessed by ATPase activity.
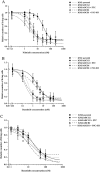
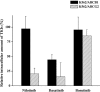
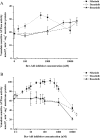
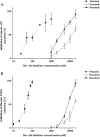
Key results: Nilotinib and dasatinib were high-affinity substrates of ABCG2, and this protein mediated an effective resistance in cancer cells against these compounds. Nilotinib and dasatinib also interacted with ABCB1, but this transporter provided resistance only against dasatinib. Neither ABCB1 nor ABCG2 induced resistance to bosutinib. At relatively higher concentrations, however, each TKI inhibited both transporters.
Conclusions and implications: A combination of in vitro assays may provide valuable preclinical information for the applicability of novel targeted anti-cancer TKIs, even in multidrug-resistant cancer. The pattern of MDR-ABC transporter-TKI interactions may also help to understand the general pharmacokinetics and toxicities of new TKIs.
Figures

References
-
- Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, et al. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. - PubMed
-
- Brozik A, Casey NP, Hegedus C, Bors A, Kozma A, Andrikovics H, et al. Reduction of Bcr-Abl function leads to erythroid differentiation of K562 cells via downregulation of ERK. Ann N Y Acad Sci. 2006;1090:344–354. - PubMed
-
- Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. - PubMed
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. - PubMed
-
- Deguchi Y, Kimura S, Ashihara E, Niwa T, Hodohara K, Fujiyama Y, et al. Comparison of imatinib, dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines. Leuk Res. 2008;32:980–983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

