Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus
- PMID: 19785757
- PMCID: PMC2761397
- DOI: 10.1186/1471-2334-9-159
Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus
Abstract
Background: Ebola and Marburg viruses cause highly lethal hemorrhagic fevers in humans. Recently, bats of multiple species have been identified as possible natural hosts of Zaire ebolavirus (ZEBOV) in Gabon and Republic of Congo, and also of marburgvirus (MARV) in Gabon and Democratic Republic of Congo.
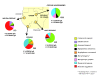
Methods: We tested 2147 bats belonging to at least nine species sampled between 2003 and 2008 in three regions of Gabon and in the Ebola epidemic region of north Congo for IgG antibodies specific for ZEBOV and MARV.
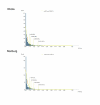
Results: Overall, IgG antibodies to ZEBOV and MARV were found in 4% and 1% of bats, respectively. ZEBOV-specific antibodies were found in six bat species (Epomops franqueti, Hypsignathus monstrosus, Myonycteris torquata, Micropteropus pusillus, Mops condylurus and Rousettus aegyptiacus), while MARV-specific antibodies were only found in Rousettus aegyptiacus and Hypsignathus monstrosus. The prevalence of MARV-specific IgG was significantly higher in R. aegyptiacus members captured inside caves than elsewhere. No significant difference in prevalence was found according to age or gender. A higher prevalence of ZEBOV-specific IgG was found in pregnant females than in non pregnant females.
Conclusion: These findings confirm that ZEBOV and MARV co-circulate in Gabon, the only country where bats infected by each virus have been found. IgG antibodies to both viruses were detected only in Rousettus aegyptiacus, suggesting that this bat species may be involved in the natural cycle of both Marburg and Ebola viruses. The presence of MARV in Gabon indicates a potential risk for a first human outbreak. Disease surveillance should be enhanced in areas near caves.
Figures

References
-
- Feldmann H, Kiley MP. Classification, structure, and replication of filoviruses. Curr Top Microbiol Immunol. 1999;235:1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

