Pediatric adverse drug events in the outpatient setting: an 11-year national analysis
- PMID: 19786435
- PMCID: PMC2975566
- DOI: 10.1542/peds.2008-3505
Pediatric adverse drug events in the outpatient setting: an 11-year national analysis
Abstract
Objective: Adverse drug events (ADEs) are a common complication of medical care, but few pediatric data are available describing the frequency or epidemiology of these events. We estimated the national incidence of pediatric ADEs requiring medical treatment, described the pediatric population seeking care for ADEs, and characterized the events in terms of patient symptoms and medications implicated.
Methods: Data were obtained from the National Center for Health Statistics, which collects information on patient visits to outpatient clinics and emergency departments throughout the United States. We analyzed data for children 0 to 18 years of age seeking medical treatment for an ADE between 1995 and 2005.
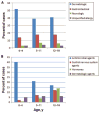
Results: The mean annual number of ADE-related visits was 585922 (95% confidence interval [CI]: 503687-668156) of which 78% occurred in outpatient clinics and 12% occurred in emergency departments. Children 0 to 4 years of age had the highest incidence of ADE-related visits, accounting for 43.2% (95% CI: 35.6%-51.2%) of visits. The most common symptom manifestations were dermatologic conditions (45.4% [95% CI: 36.9%-54.1%]) and gastrointestinal symptoms (16.5% [95% CI: 11.1%-23.8%]). The medication classes most frequently implicated in an ADE were antimicrobial agents (27.5% [95% CI: 21.5%-34.5%]), central nervous system agents (6.5% [95% CI: 4.0%-10.5%]), and hormones (6.1% [95% CI: 3.1%-11.6%]). While ADEs related to antimicrobial agents were most common among children 0 to 4 years old and decreased in frequency among older children, ADEs resulting from central nervous system agents and hormones increased in frequency among children 5 to 11 and 12 to 18 years old.
Conclusions: ADEs result in a substantial number of health care visits, particularly in outpatient clinics. The incidence of ADEs and medications implicated vary by age, indicating that age-specific approaches for monitoring and preventing ADEs may be most effective.
Figures
References
-
- Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 summary. Adv Data. 2007;387:1–39. - PubMed
-
- National Center for Health Statistics. Health, United States, 2007, with chartbook on trends in the health of Americans. [Accessed August 3, 2009]. Available at: www.cdc.gov/nchs/hus.htm. - PubMed
-
- Slone Epidemiology Center. Patterns of medication use in the United States, 2006: a report from the Slone Survey. [Accessed August 3, 2009]. Available at: www.bu.edu/slone/SloneSurvey/SloneSurvey.htm.
-
- Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. - PubMed
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical