Autophagy promotes synapse development in Drosophila
- PMID: 19786572
- PMCID: PMC2762098
- DOI: 10.1083/jcb.200907109
Autophagy promotes synapse development in Drosophila
Abstract
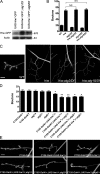
Autophagy, a lysosome-dependent degradation mechanism, mediates many biological processes, including cellular stress responses and neuroprotection. In this study, we demonstrate that autophagy positively regulates development of the Drosophila melanogaster larval neuromuscular junction (NMJ). Autophagy induces an NMJ overgrowth phenotype closely resembling that of highwire (hiw), an E3 ubiquitin ligase mutant. Moreover, like hiw, autophagy-induced NMJ overgrowth is suppressed by wallenda (wnd) and by a dominant-negative c-Jun NH(2)-terminal kinase (bsk(DN)). We show that autophagy promotes NMJ growth by reducing Hiw levels. Thus, autophagy and the ubiquitin-proteasome system converge in regulating synaptic development. Because autophagy is triggered in response to many environmental cues, our findings suggest that it is perfectly positioned to link environmental conditions with synaptic growth and plasticity.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

