Paracrine induction of endothelium by tumor exosomes
- PMID: 19786948
- PMCID: PMC3316485
- DOI: 10.1038/labinvest.2009.94
Paracrine induction of endothelium by tumor exosomes
Abstract
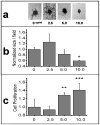
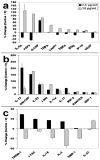
Cancers use a nanoscale messenger system known as exosomes to communicate with surrounding tissues and immune cells. However, the functional relationship between tumor exosomes, endothelial signaling, angiogenesis, and metastasis is poorly understood. Herein, we describe a standardized approach for defining the angiogenic potential of isolated exosomes. We created a powerful technique to rapidly and efficiently isolate and track exosomes for study using dynamic light scattering in conjunction with fluorescent exosome labeling. With these methods, melanoma exosomes were observed to interact with and influence endothelial tubule morphology as well as move between endothelial tubule cells by means of tunneling nanotube structures. Melanoma exosomes also were observed to rapidly stimulate the production of endothelial spheroids and endothelial sprouts in a dose-dependent manner. In concert, tumor exosomes simultaneously elicited paracrine endothelial signaling by regulation of certain inflammatory cytokines. These data suggest that, tumor exosomes can promote endothelial angiogenic responses, which could contribute to tumor metastatic potential.
Figures



Comment in
-
Inside lab invest.Lab Invest. 2009 Nov;89(11):1190-1. doi: 10.1038/labinvest.2009.114. Lab Invest. 2009. PMID: 19861966 No abstract available.
References
-
- Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. - PubMed
-
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80–88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

