Modifications of antiepileptic drugs for improved tolerability and efficacy
- PMID: 19787095
- PMCID: PMC2746576
Modifications of antiepileptic drugs for improved tolerability and efficacy
Abstract
Introduction: A large number of antiepileptic drugs (AEDs) are available today, but they may not be satisfactory regarding clinical efficacy, tolerance, toxicity or pharmacokinetic properties. The purpose of this review is to focus upon the rationale behind the chemical modifications of several recently marketed AEDs or drugs in development and to categorize them according to the main purposes for the improvements: better efficacy or tolerability accompanied by improved pharmacokinetic properties.
Material and method: AEDs that have been chemically modified to new derivatives during the last years are reviewed based on recent publications and PubMed-searches.
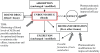
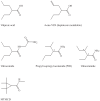
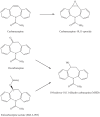
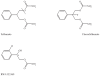
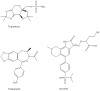
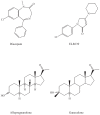
Results and discussion: Improvement in pharmacokinetic parameters may affect both tolerability and efficacy. Modifications to improve tolerability include various valproate analogues, divided into aliphatic amides, cyclic derivatives or amino acid conjugates. Furthermore, there are the carbamazepine analogues oxcarbazepine and eslicarbazepine, the felbamate analogues fluorofelbamate and carisbamate (RWJ 33369), and the lamotrigine analogue JZP-4. The levetiracetam analogues brivaracetam and seletracetam and the derivatives of gabapentin, pregabalin and XP13512, have improved selectivity compared to their parent compounds. Other new drugs have new mechanisms of action related to GABA and glutamate receptors; the glutamate antagonists like topiramate (talampanel and NS-1209), and GABA(A) receptor agonists, benzodiazepine or progesterone analogues (ELB-139 and ganaxolone).
Conclusion: Further challenges for development of new AEDs include investigations of target molecules affected by pathophysiological processes and detailed structure-activity relationships with focus on stereoselectivity. These potential drugs may become of importance in future drug therapy in epilepsy and other CNS disorders.
Keywords: antiepileptic drugs; chemical modification; efficacy; monitoring; pharmacodynamics; pharmacokinetics; tolerability.
Figures

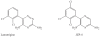
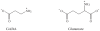


References
-
- Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res. 2005;63:141–9. - PubMed
-
- Aldenkamp A, Vigevano F, Arzimanoglou A, et al. Role of valproate across the ages. Treatment of epilepsy in children. Acta. Neurol. Scand. 2006;114(suppl 184):1–13. - PubMed
-
- Anmann B, Grünze H, Vieta E, et al. Antiepileptic drugs and mood stability. Clin. EEG Neurosci. 2007;38:116–23. - PubMed
-
- Anmann B, Grünze H. Neurochemical underpinnings in bipolar disorder and epilepsy. Epilepsia. 2005;46:26–30. - PubMed
-
- Bailie T. Metabolism of valproate to heaptotoxic intermediates. Pharm. Weekbl. Sci. 1992;14:122–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
