Analysis of risk factors for central venous port failure in cancer patients
- PMID: 19787834
- PMCID: PMC2754519
- DOI: 10.3748/wjg.15.4709
Analysis of risk factors for central venous port failure in cancer patients
Abstract
Aim: To analyze the risk factors for central port failure in cancer patients administered chemotherapy, using univariate and multivariate analyses.
Methods: A total of 1348 totally implantable venous access devices (TIVADs) were implanted into 1280 cancer patients in this cohort study. A Cox proportional hazard model was applied to analyze risk factors for failure of TIVADs. Log-rank test was used to compare actuarial survival rates. Infection, thrombosis, and surgical complication rates (chi(2) test or Fisher's exact test) were compared in relation to the risk factors.
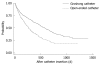
Results: Increasing age, male gender and open-ended catheter use were significant risk factors reducing survival of TIVADs as determined by univariate and multivariate analyses. Hematogenous malignancy decreased the survival time of TIVADs; this reduction was not statistically significant by univariate analysis [hazard ratio (HR) = 1.336, 95% CI: 0.966-1.849, P = 0.080)]. However, it became a significant risk factor by multivariate analysis (HR = 1.499, 95% CI: 1.079-2.083, P = 0.016) when correlated with variables of age, sex and catheter type. Close-ended (Groshong) catheters had a lower thrombosis rate than open-ended catheters (2.5% vs 5%, P = 0.015). Hematogenous malignancy had higher infection rates than solid malignancy (10.5% vs 2.5%, P < 0.001).
Conclusion: Increasing age, male gender, open-ended catheters and hematogenous malignancy were risk factors for TIVAD failure. Close-ended catheters had lower thrombosis rates and hematogenous malignancy had higher infection rates.
Figures
References
-
- Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973;136:602–606. - PubMed
-
- Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979;148:871–875. - PubMed
-
- Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712. - PubMed
-
- Biffi R, Corrado F, de Braud F, de Lucia F, Scarpa D, Testori A, Orsi F, Bellomi M, Mauri S, Aapro M, et al. Long-term, totally implantable central venous access ports connected to a Groshong catheter for chemotherapy of solid tumours: experience from 178 cases using a single type of device. Eur J Cancer. 1997;33:1190–1194. - PubMed
-
- Biffi R, De Braud F, Orsi F, Pozzi S, Arnaldi P, Goldhirsch A, Rotmensz N, Robertson C, Bellomi M, Andreoni B. A randomized, prospective trial of central venous ports connected to standard open-ended or Groshong catheters in adult oncology patients. Cancer. 2001;92:1204–1212. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources