Coordination chemistry of bacterial metal transport and sensing
- PMID: 19788177
- PMCID: PMC2783614
- DOI: 10.1021/cr900077w
Coordination chemistry of bacterial metal transport and sensing
Figures








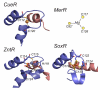
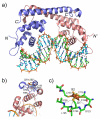
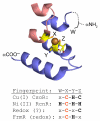




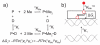
References
-
- Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. University Science Books; Mill Valley, CA: 1994.
-
- Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ. Nature. 2008;455:1138. - PubMed
-
- Waldron KJ, Robinson NJ. Nat. Rev. Microbiol. 2009;7:25. - PubMed
-
- Duran RV, Hervas M, De La Rosa MA, Navarro JA. J. Biol. Chem. 2004;279:7229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

