Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS
- PMID: 19788312
- PMCID: PMC2756691
- DOI: 10.1021/ac901154s
Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS
Abstract
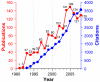
Understanding as much as possible about proteins in the shortest amount of time has long been a goal of hydrogen exchange (HX) MS. Recent technological advances have led to improvements in the technique, but has this goal yet been achieved? (To listen to a podcast about this Feature, please go to the Analytical Chemistry Web site at pubs.acs.org/journal/ancham.).
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

