Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection
- PMID: 19788504
- PMCID: PMC2860022
- DOI: 10.1111/j.1600-6143.2009.02826.x
Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection
Abstract
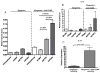
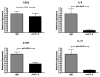
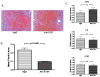
Cardiac transplantation is an effective treatment for multiple types of heart failure refractive to therapy. Although immunosuppressive therapeutics have increased survival rates within the first year posttransplant, chronic rejection (CR) remains a significant barrier to long-term graft survival. Indicators of CR include patchy interstitial fibrosis, vascular occlusion and progressive loss of graft function. Multiple factors have been implicated in the onset and progression of CR, including TGFbeta, IL-6 and connective tissue growth factor (CTGF). While associated with CR, the role of CTGF in CR and the factors necessary for CTGF induction in vivo are not understood. To this end, we utilized forced expression and neutralizing antibody approaches. Transduction of allografts with CTGF significantly increased fibrotic tissue development, though not to levels observed with TGFbeta transduction. Further, intragraft CTGF expression was inhibited by IL-6 neutralization whereas TGFbeta expression remained unchanged, indicating that IL-6 effects may potentiate TGFbeta-mediated induction of CTGF. Finally, neutralizing CTGF significantly reduced graft fibrosis without reducing TGFbeta and IL-6 expression levels. These findings indicate that CTGF functions as a downstream mediator of fibrosis in CR, and that CTGF neutralization may ameliorate fibrosis and hypertrophy associated with CR.
Figures
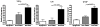
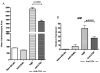
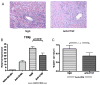
Comment in
-
Allograft fibrosis--unmasking the players at the dance.Am J Transplant. 2010 Feb;10(2):201-2. doi: 10.1111/j.1600-6143.2009.02926.x. Am J Transplant. 2010. PMID: 20422744 No abstract available.
Similar articles
-
TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection.Immunotherapy. 2010 Jul;2(4):511-20. doi: 10.2217/imt.10.33. Immunotherapy. 2010. PMID: 20636005 Free PMC article. Review.
-
TGFbeta neutralization within cardiac allografts by decorin gene transfer attenuates chronic rejection.J Immunol. 2009 Dec 1;183(11):7307-13. doi: 10.4049/jimmunol.0902736. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917705 Free PMC article.
-
Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection.J Immunol. 2009 Dec 1;183(11):7297-306. doi: 10.4049/jimmunol.0902446. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917689 Free PMC article.
-
Transforming growth factor beta-induced connective tissue growth factor and chronic allograft rejection.Am J Transplant. 2006 May;6(5 Pt 1):959-66. doi: 10.1111/j.1600-6143.2006.01292.x. Am J Transplant. 2006. PMID: 16611331
-
The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology.Biochem Cell Biol. 2003 Dec;81(6):355-63. doi: 10.1139/o03-069. Biochem Cell Biol. 2003. PMID: 14663501 Review.
Cited by
-
Predictive value of serum CTRP9 and STIM1 for restenosis after cerebrovascular stent implantation and its relationship with vasoactive substances and inflammatory cytokines.Exp Ther Med. 2020 Sep;20(3):2617-2622. doi: 10.3892/etm.2020.9104. Epub 2020 Aug 6. Exp Ther Med. 2020. PMID: 32793308 Free PMC article.
-
Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection.Eur Heart J. 2013 Jul;34(25):1930-41. doi: 10.1093/eurheartj/ehs375. Epub 2012 Nov 8. Eur Heart J. 2013. PMID: 23139380 Free PMC article.
-
Th17 cells and transplant acceptance.Transplantation. 2010 Nov 15;90(9):945-8. doi: 10.1097/TP.0b013e3181f5c3de. Transplantation. 2010. PMID: 20838278 Free PMC article. Review.
-
TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection.Immunotherapy. 2010 Jul;2(4):511-20. doi: 10.2217/imt.10.33. Immunotherapy. 2010. PMID: 20636005 Free PMC article. Review.
-
Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease.Fibrogenesis Tissue Repair. 2011 Feb 1;4(1):4. doi: 10.1186/1755-1536-4-4. Fibrogenesis Tissue Repair. 2011. PMID: 21284856 Free PMC article.
References
-
- Orosz CG, Pelletier RP. Chronic remodeling pathology in grafts. Curr Opin Immunol. 1997;9(5):676–680. - PubMed
-
- Paul LC. Current knowledge of the pathogenesis of chronic allograft dysfunction. Transplant Proc. 1999;31(4):1793–1795. - PubMed
-
- Waaga AM, Gasser M, Laskowski I, Tilney NL. Mechanisms of chronic rejection. Curr Opin Immunol. 2000;12(5):517–521. - PubMed
-
- Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol. 2000;20(2):126–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

