PKA and Epac cooperate to augment bradykinin-induced interleukin-8 release from human airway smooth muscle cells
- PMID: 19788733
- PMCID: PMC2764632
- DOI: 10.1186/1465-9921-10-88
PKA and Epac cooperate to augment bradykinin-induced interleukin-8 release from human airway smooth muscle cells
Abstract
Background: Airway smooth muscle contributes to the pathogenesis of pulmonary diseases by secreting inflammatory mediators such as interleukin-8 (IL-8). IL-8 production is in part regulated via activation of Gq-and Gs-coupled receptors. Here we study the role of the cyclic AMP (cAMP) effectors protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac1 and Epac2) in the bradykinin-induced IL-8 release from a human airway smooth muscle cell line and the underlying molecular mechanisms of this response.
Methods: IL-8 release was assessed via ELISA under basal condition and after stimulation with bradykinin alone or in combination with fenoterol, the Epac activators 8-pCPT-2'-O-Me-cAMP and Sp-8-pCPT-2'-O-Me-cAMPS, the PKA activator 6-Bnz-cAMP and the cGMP analog 8-pCPT-2'-O-Me-cGMP. Where indicated, cells were pre-incubated with the pharmacological inhibitors Clostridium difficile toxin B-1470 (GTPases), U0126 (extracellular signal-regulated kinases ERK1/2) and Rp-8-CPT-cAMPS (PKA). The specificity of the cyclic nucleotide analogs was confirmed by measuring phosphorylation of the PKA substrate vasodilator-stimulated phosphoprotein. GTP-loading of Rap1 and Rap2 was evaluated via pull-down technique. Expression of Rap1, Rap2, Epac1 and Epac2 was assessed via western blot. Downregulation of Epac protein expression was achieved by siRNA. Unpaired or paired two-tailed Student's t test was used.
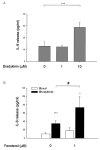
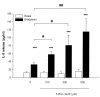
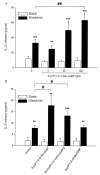
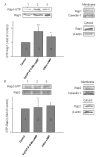
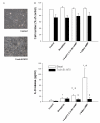
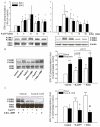
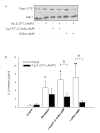
Results: The beta2-agonist fenoterol augmented release of IL-8 by bradykinin. The PKA activator 6-Bnz-cAMP and the Epac activator 8-pCPT-2'-O-Me-cAMP significantly increased bradykinin-induced IL-8 release. The hydrolysis-resistant Epac activator Sp-8-pCPT-2'-O-Me-cAMPS mimicked the effects of 8-pCPT-2'-O-Me-cAMP, whereas the negative control 8-pCPT-2'-O-Me-cGMP did not. Fenoterol, forskolin and 6-Bnz-cAMP induced VASP phosphorylation, which was diminished by the PKA inhibitor Rp-8-CPT-cAMPS. 6-Bnz-cAMP and 8-pCPT-2'-O-Me-cAMP induced GTP-loading of Rap1, but not of Rap2. Treatment of the cells with toxin B-1470 and U0126 significantly reduced bradykinin-induced IL-8 release alone or in combination with the activators of PKA and Epac. Interestingly, inhibition of PKA by Rp-8-CPT-cAMPS and silencing of Epac1 and Epac2 expression by specific siRNAs largely decreased activation of Rap1 and the augmentation of bradykinin-induced IL-8 release by both PKA and Epac.
Conclusion: Collectively, our data suggest that PKA, Epac1 and Epac2 act in concert to modulate inflammatory properties of airway smooth muscle via signaling to the Ras-like GTPase Rap1 and to ERK1/2.
Figures





References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. - PubMed
-
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. - PubMed
-
- Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

