Protein and energy metabolism in type 1 diabetes
- PMID: 19788950
- PMCID: PMC2822109
- DOI: 10.1016/j.clnu.2009.09.001
Protein and energy metabolism in type 1 diabetes
Abstract
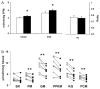
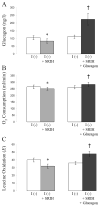
Profound metabolic changes occur in people with type 1 diabetes mellitus during insulin deprivation. These include an increase in basal energy expenditure and reduced mitochondrial function. In addition, protein metabolism is significantly affected during insulin deprivation. A greater increase in whole-body protein breakdown than protein synthesis occurs resulting in a net protein loss. During insulin deprivation the splanchnic bed has a net protein accretion which accounts for the total increase in whole-body protein synthesis while muscle is in a net catabolic state.
Copyright 2009 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures
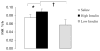

References
-
- Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47(11):1748–56. - PubMed
-
- Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes. 2007;56(11):2683–9. - PubMed
-
- Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27(1):13–6. - PubMed
-
- Chhibber VL, Soriano C, Tayek JA. Effects of low-dose and high-dose glucagon on glucose production and gluconeogenesis in humans. Metabolism. 2000;49(1):39–46. - PubMed
-
- Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987;64(5):896–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

