Lymph node-targeted immunotherapy mediates potent immunity resulting in regression of isolated or metastatic human papillomavirus-transformed tumors
- PMID: 19789304
- PMCID: PMC2756704
- DOI: 10.1158/1078-0432.CCR-09-0645
Lymph node-targeted immunotherapy mediates potent immunity resulting in regression of isolated or metastatic human papillomavirus-transformed tumors
Abstract
Purpose: The goal of this study was to investigate the therapeutic potential of a novel immunotherapy strategy resulting in immunity to localized or metastatic human papillomavirus 16-transformed murine tumors.
Experimental design: Animals bearing E7-expressing tumors were coimmunized by lymph node injection with E7 49-57 antigen and TLR3-ligand (synthetic dsRNA). Immune responses were measured by flow cytometry and antitumor efficacy was evaluated by tumor size and survival. In situ cytotoxicity assays and identification of tumor-infiltrating lymphocytes and T regulatory cells were used to assess the mechanisms of treatment resistance in bulky disease. Chemotherapy with cyclophosphamide was explored to augment immunotherapy in late-stage disease.
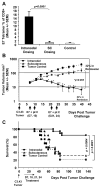
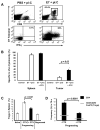
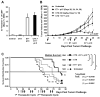
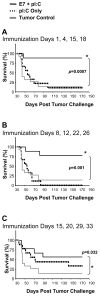
Results: In therapeutic and prophylactic settings, immunization resulted in a considerable expansion of E7 49-57 antigen-specific T lymphocytes in the range of 1/10 CD8(+) T cells. The resulting immunity was effective in suppressing disease progression and mortality in a pulmonary metastatic disease model. Therapeutic immunization resulted in control of isolated tumors up to a certain volume, and correlated with antitumor immune responses measured in blood. In situ analysis showed that within bulky tumors, T-cell function was affected by negative regulatory mechanisms linked to an increase in T regulatory cells and could be overcome by cyclophosphamide treatment in conjunction with immunization.
Conclusions: This study highlights a novel cancer immunotherapy platform with potential for translatability to the clinic and suggests its potential usefulness for controlling metastatic disease, solid tumors of limited size, or larger tumors when combined with cytotoxic agents that reduce the number of tumor-infiltrating T regulatory cells.
Figures

References
-
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. - PubMed
-
- Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. - PubMed
-
- Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

