Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT
- PMID: 19789626
- PMCID: PMC2746281
- DOI: 10.1371/journal.pone.0007258
Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT
Abstract
Background: The stem cell factor receptor, KIT, is a target for the treatment of cancer, mastocytosis, and inflammatory diseases. Here, we characterise the in vitro and in vivo profiles of masitinib (AB1010), a novel phenylaminothiazole-type tyrosine kinase inhibitor that targets KIT.
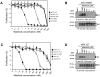
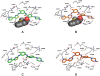
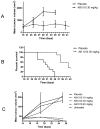
Methodology/principal findings: In vitro, masitinib had greater activity and selectivity against KIT than imatinib, inhibiting recombinant human wild-type KIT with an half inhibitory concentration (IC(50)) of 200+/-40 nM and blocking stem cell factor-induced proliferation and KIT tyrosine phosphorylation with an IC(50) of 150+/-80 nM in Ba/F3 cells expressing human or mouse wild-type KIT. Masitinib also potently inhibited recombinant PDGFR and the intracellular kinase Lyn, and to a lesser extent, fibroblast growth factor receptor 3. In contrast, masitinib demonstrated weak inhibition of ABL and c-Fms and was inactive against a variety of other tyrosine and serine/threonine kinases. This highly selective nature of masitinib suggests that it will exhibit a better safety profile than other tyrosine kinase inhibitors; indeed, masitinib-induced cardiotoxicity or genotoxicity has not been observed in animal studies. Molecular modelling and kinetic analysis suggest a different mode of binding than imatinib, and masitinib more strongly inhibited degranulation, cytokine production, and bone marrow mast cell migration than imatinib. Furthermore, masitinib potently inhibited human and murine KIT with activating mutations in the juxtamembrane domain. In vivo, masitinib blocked tumour growth in mice with subcutaneous grafts of Ba/F3 cells expressing a juxtamembrane KIT mutant.
Conclusions: Masitinib is a potent and selective tyrosine kinase inhibitor targeting KIT that is active, orally bioavailable in vivo, and has low toxicity.
Conflict of interest statement
Figures
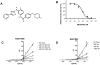



Similar articles
-
Masitinib (AB1010), from canine tumor model to human clinical development: where we are?Crit Rev Oncol Hematol. 2014 Jul;91(1):98-111. doi: 10.1016/j.critrevonc.2013.12.011. Epub 2013 Dec 17. Crit Rev Oncol Hematol. 2014. PMID: 24405856 Review.
-
Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies.Cancer Res. 2006 Jan 1;66(1):473-81. doi: 10.1158/0008-5472.CAN-05-2050. Cancer Res. 2006. PMID: 16397263
-
Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis.Blood. 2006 Jul 1;108(1):286-91. doi: 10.1182/blood-2005-10-3969. Epub 2006 Jan 24. Blood. 2006. PMID: 16434489
-
An evaluation of masitinib for treating systemic mastocytosis.Expert Opin Pharmacother. 2019 Sep;20(13):1539-1550. doi: 10.1080/14656566.2019.1645121. Epub 2019 Aug 5. Expert Opin Pharmacother. 2019. PMID: 31381378 Review.
-
Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 c-kit.Leuk Res. 2006 Nov;30(11):1365-70. doi: 10.1016/j.leukres.2006.04.005. Epub 2006 Jun 23. Leuk Res. 2006. PMID: 16797704
Cited by
-
Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression.Eur J Haematol. 2013 Feb;90(2):89-98. doi: 10.1111/ejh.12043. Eur J Haematol. 2013. PMID: 23181448 Free PMC article. Review.
-
Kinase inhibitors as potential agents in the treatment of multiple myeloma.Oncotarget. 2016 Dec 6;7(49):81926-81968. doi: 10.18632/oncotarget.10745. Oncotarget. 2016. PMID: 27655636 Free PMC article. Review.
-
Mast cells as cellular sensors in inflammation and immunity.Front Immunol. 2011 Sep 6;2:37. doi: 10.3389/fimmu.2011.00037. eCollection 2011. Front Immunol. 2011. PMID: 22566827 Free PMC article.
-
Drugging Hijacked Kinase Pathways in Pediatric Oncology: Opportunities and Current Scenario.Pharmaceutics. 2023 Feb 16;15(2):664. doi: 10.3390/pharmaceutics15020664. Pharmaceutics. 2023. PMID: 36839989 Free PMC article. Review.
-
Target Therapies for Systemic Mastocytosis: An Update.Pharmaceuticals (Basel). 2022 Jun 11;15(6):738. doi: 10.3390/ph15060738. Pharmaceuticals (Basel). 2022. PMID: 35745657 Free PMC article. Review.
References
-
- Roskoski R., Jr Signaling by Kit protein-tyrosine kinase - the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. - PubMed
-
- Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. - PubMed
-
- Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–40. - PubMed
-
- Tests U. Kit mutations in cancer and their treatment with protein kinase inhibitors. Drugs Fut. 2008;33:161–174.
-
- Lennarttson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and Oncogenic Forms of the Receptor Tyrosine Kinase Kit. Stem Cells. 2005;23:16–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

