Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis
- PMID: 19790060
- PMCID: PMC3757557
- DOI: 10.1002/art.24814
Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis
Abstract
Objective: Osteopontin (OPN) is a proinflammatory cytokine that plays an important role in the pathogenesis of rheumatoid arthritis (RA). OPN can be cleaved by thrombin, resulting in OPN-R and exposing the cryptic C-terminal alpha4beta1 and alpha9beta1 integrin-binding motif (SVVYGLR). Thrombin-activatable carboxypeptidase B (CPB), also called thrombin-activatable fibrinolysis inhibitor, removes the C-terminal arginine from OPN-R, generating OPN-L and abrogating its enhanced cell binding. We undertook this study to investigate the roles of OPN-R and OPN-L in synoviocyte adhesion, which contributes to the formation of invasive pannus, and in neutrophil survival, which affects inflammatory infiltrates in RA.
Methods: Using specifically developed enzyme-linked immunosorbent assays, we tested the synovial fluid of patients with RA, osteoarthritis (OA), and psoriatic arthritis (PsA) to determine OPN-R, OPN-L, and full-length OPN (OPN-FL) levels.
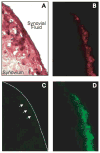
Results: Elevated levels of OPN-R and OPN-L were found in synovial fluid samples from RA patients, but not in samples from OA or PsA patients. Increased levels of OPN-R and OPN-L correlated with increased levels of multiple inflammatory cytokines, including tumor necrosis factor alpha and interleukin-6. Immunohistochemical analyses revealed robust expression of OPN-FL, but only minimal expression of OPN-R, in RA synovium, suggesting that cleaved OPN is released into synovial fluid. In cellular assays, OPN-FL, and to a lesser extent OPN-R and OPN-L, had an antiapoptotic effect on neutrophils. OPN-R augmented RA fibroblast-like synoviocyte binding mediated by SVVYGLR binding to alpha4beta1, whereas OPN-L did not.
Conclusion: Thrombin activation of OPN (resulting in OPN-R) and its subsequent inactivation by thrombin-activatable CPB (generating OPN-L) occurs locally within inflamed joints in RA. Our data suggest that thrombin-activatable CPB plays a central homeostatic role in RA by regulating neutrophil viability and reducing synoviocyte adhesion.
Conflict of interest statement
Conflict of interest: None
Figures




Similar articles
-
Role of osteopontin in rheumatoid arthritis.Rheumatol Int. 2015 Apr;35(4):589-95. doi: 10.1007/s00296-014-3122-z. Epub 2014 Aug 28. Rheumatol Int. 2015. PMID: 25163663 Review.
-
Thrombin-cleaved osteopontin in synovial fluid of subjects with rheumatoid arthritis.J Rheumatol. 2009 Feb;36(2):240-5. doi: 10.3899/jrheum.080753. Epub 2009 Jan 22. J Rheumatol. 2009. PMID: 19208558
-
Enhanced local production of osteopontin in rheumatoid joints.J Rheumatol. 2002 Oct;29(10):2061-7. J Rheumatol. 2002. PMID: 12375312
-
Specifically modified osteopontin in rheumatoid arthritis fibroblast-like synoviocytes supports interaction with B cells and enhances production of interleukin-6.Arthritis Rheum. 2009 Dec;60(12):3591-601. doi: 10.1002/art.25020. Arthritis Rheum. 2009. PMID: 19950274
-
Osteopontin: A Bone-Derived Protein Involved in Rheumatoid Arthritis and Osteoarthritis Immunopathology.Biomolecules. 2023 Mar 9;13(3):502. doi: 10.3390/biom13030502. Biomolecules. 2023. PMID: 36979437 Free PMC article. Review.
Cited by
-
Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis.Nat Rev Rheumatol. 2016 Oct;12(10):580-92. doi: 10.1038/nrrheum.2016.136. Epub 2016 Aug 19. Nat Rev Rheumatol. 2016. PMID: 27539668 Free PMC article. Review.
-
Thrombin Cleavage of Osteopontin and the Host Anti-Tumor Immune Response.Cancers (Basel). 2023 Jul 3;15(13):3480. doi: 10.3390/cancers15133480. Cancers (Basel). 2023. PMID: 37444590 Free PMC article. Review.
-
Role of osteopontin in rheumatoid arthritis.Rheumatol Int. 2015 Apr;35(4):589-95. doi: 10.1007/s00296-014-3122-z. Epub 2014 Aug 28. Rheumatol Int. 2015. PMID: 25163663 Review.
-
The embryonic type of SPP1 transcriptional regulation is re-activated in glioblastoma.Oncotarget. 2017 Mar 7;8(10):16340-16355. doi: 10.18632/oncotarget.14092. Oncotarget. 2017. PMID: 28030801 Free PMC article.
-
Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis.Arterioscler Thromb Vasc Biol. 2011 Aug;31(8):1821-33. doi: 10.1161/ATVBAHA.111.230011. Epub 2011 May 19. Arterioscler Thromb Vasc Biol. 2011. PMID: 21597007 Free PMC article.
References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

