Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study
- PMID: 19790071
- PMCID: PMC2842939
- DOI: 10.1002/art.24803
Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study
Abstract
Objective: Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by unpredictable flares of disease activity and irreversible damage to multiple organ systems. An earlier study showed that SLE patients carrying an interferon (IFN) gene expression signature in blood have elevated serum levels of IFN-regulated chemokines. These chemokines were associated with more-severe and active disease and showed promise as SLE disease activity biomarkers. This study was designed to validate IFN-regulated chemokines as biomarkers of SLE disease activity in 267 SLE patients followed up longitudinally.
Methods: To validate the potential utility of serum chemokine levels as biomarkers of disease activity, we measured serum levels of CXCL10 (IFNgamma-inducible 10-kd protein), CCL2 (monocyte chemotactic protein 1), and CCL19 (macrophage inflammatory protein 3beta) in an independent cohort of 267 SLE patients followed up longitudinally over 1 year (1,166 total clinic visits).
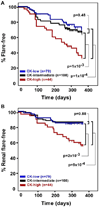
Results: Serum chemokine levels correlated with lupus activity at the current visit (P = 2 x 10(-10)), rising at the time of SLE flare (P = 2 x 10(-3)) and decreasing as disease remitted (P = 1 x 10(-3)); they also performed better than the currently available laboratory tests. Chemokine levels measured at a single baseline visit in patients with a Systemic Lupus Erythematosus Disease Activity Index of < or =4 were predictive of lupus flare over the ensuing year (P = 1 x 10(-4)).
Conclusion: Monitoring serum chemokine levels in SLE may improve the assessment of current disease activity, the prediction of future disease flares, and the overall clinical decision-making.
Conflict of interest statement
Dr. Behrens reports being an employee of Genentech and having an equity interest in the company; Dr. Gregersen reports serving on the Abbott Scholar Award Advisory Committee and receiving honoraria from Biogen Idec, Genentech, and Roche Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
Figures




References
-
- Wallace DJ, Hahn BH, editors. 7th ed. Baltimore: Williams & Wilkins; 2007.
-
- Esdaile JM, Abrahamowicz M, Joseph L, MacKenzie T, Li Y, Danoff D. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996;39(3):370–378. - PubMed
-
- Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum. 2004;50(7):2048–2065. - PubMed
-
- Kavanaugh A. The utility of immunologic laboratory tests in patients with rheumatic diseases. Arthritis Rheum. 2001;44(10):2221–2223. - PubMed
-
- Liu CC, Manzi S, Ahearn JM. Biomarkers for systemic lupus erythematosus: a review and perspective. Curr Opin Rheumatol. 2005;17(5):543–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

