Progress and challenges in the automated construction of Markov state models for full protein systems
- PMID: 19791846
- PMCID: PMC2766407
- DOI: 10.1063/1.3216567
Progress and challenges in the automated construction of Markov state models for full protein systems
Abstract
Markov state models (MSMs) are a powerful tool for modeling both the thermodynamics and kinetics of molecular systems. In addition, they provide a rigorous means to combine information from multiple sources into a single model and to direct future simulations/experiments to minimize uncertainties in the model. However, constructing MSMs is challenging because doing so requires decomposing the extremely high dimensional and rugged free energy landscape of a molecular system into long-lived states, also called metastable states. Thus, their application has generally required significant chemical intuition and hand-tuning. To address this limitation we have developed a toolkit for automating the construction of MSMs called MSMBUILDER (available at https://simtk.org/home/msmbuilder). In this work we demonstrate the application of MSMBUILDER to the villin headpiece (HP-35 NleNle), one of the smallest and fastest folding proteins. We show that the resulting MSM captures both the thermodynamics and kinetics of the original molecular dynamics of the system. As a first step toward experimental validation of our methodology we show that our model provides accurate structure prediction and that the longest timescale events correspond to folding.
Figures
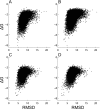
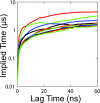


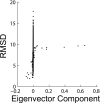




Similar articles
-
Using generalized ensemble simulations and Markov state models to identify conformational states.Methods. 2009 Oct;49(2):197-201. doi: 10.1016/j.ymeth.2009.04.013. Epub 2009 May 4. Methods. 2009. PMID: 19410002 Free PMC article.
-
MSMBuilder: Statistical Models for Biomolecular Dynamics.Biophys J. 2017 Jan 10;112(1):10-15. doi: 10.1016/j.bpj.2016.10.042. Biophys J. 2017. PMID: 28076801 Free PMC article.
-
MSMExplorer: visualizing Markov state models for biomolecule folding simulations.Bioinformatics. 2013 Apr 1;29(7):950-2. doi: 10.1093/bioinformatics/btt051. Epub 2013 Jan 30. Bioinformatics. 2013. PMID: 23365411
-
Markov state models provide insights into dynamic modulation of protein function.Acc Chem Res. 2015 Feb 17;48(2):414-22. doi: 10.1021/ar5002999. Epub 2015 Jan 3. Acc Chem Res. 2015. PMID: 25625937 Free PMC article. Review.
-
Simulating the peptide folding kinetic related spectra based on the Markov State Model.Adv Exp Med Biol. 2014;805:199-220. doi: 10.1007/978-3-319-02970-2_9. Adv Exp Med Biol. 2014. PMID: 24446363 Review.
Cited by
-
Event detection and sub-state discovery from biomolecular simulations using higher-order statistics: application to enzyme adenylate kinase.Proteins. 2012 Nov;80(11):2536-51. doi: 10.1002/prot.24135. Epub 2012 Aug 8. Proteins. 2012. PMID: 22733562 Free PMC article.
-
How kinetics within the unfolded state affects protein folding: an analysis based on markov state models and an ultra-long MD trajectory.J Phys Chem B. 2013 Oct 24;117(42):12787-99. doi: 10.1021/jp401962k. Epub 2013 Jun 13. J Phys Chem B. 2013. PMID: 23705683 Free PMC article.
-
GraphVAMPNet, using graph neural networks and variational approach to Markov processes for dynamical modeling of biomolecules.J Chem Phys. 2022 May 14;156(18):184103. doi: 10.1063/5.0085607. J Chem Phys. 2022. PMID: 35568532 Free PMC article.
-
Complete reconstruction of an enzyme-inhibitor binding process by molecular dynamics simulations.Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10184-9. doi: 10.1073/pnas.1103547108. Epub 2011 Jun 6. Proc Natl Acad Sci U S A. 2011. PMID: 21646537 Free PMC article.
-
Locking the active conformation of c-Src kinase through the phosphorylation of the activation loop.J Mol Biol. 2014 Jan 23;426(2):423-35. doi: 10.1016/j.jmb.2013.10.001. Epub 2013 Oct 6. J Mol Biol. 2014. PMID: 24103328 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

