Unique molecular characteristics of pediatric myxopapillary ependymoma
- PMID: 19793339
- PMCID: PMC2871180
- DOI: 10.1111/j.1750-3639.2009.00333.x
Unique molecular characteristics of pediatric myxopapillary ependymoma
Abstract
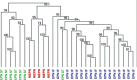
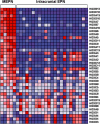
Myxopapillary ependymoma (MEPN) generally can be cured by gross total surgical resection and usually manifest a favorable prognosis. However, surgery is less curative in tumors that are large, multifocal or extend outside the thecal sac. Late recurrences may occur, particularly in pediatric patients. The role of adjuvant therapy is unclear in the clinical management of recurrent tumors. Clinical trial design requires a better understanding of tumor biology. Unique molecular features of MEPN were investigated by using microarray technology to compare the gene expression of five pediatric MEPN to 24 pediatric intracranial ependymoma (EPN). The upregulation of three genes of interest, homeobox B13 (HOXB13), neurofilament, light polypeptide (NEFL) and PDGFR alpha, was further studied by immunohistochemistry in a larger cohort that included adult MEPN and EPN specimens. Protein expression in MEPN was compared to subependymoma, spinal EPN, intracranial EPN and normal fetal and adult ependyma. Immunoreactivity for HOXB13, NEFL and PDGFR alpha was strongest in MEPN and virtually absent in subependymoma. Spinal and intracranial EPN generally expressed weak or focal staining. MEPN manifests unique gene and protein expression patterns compared to other EPNs. Aberrant expression of HOXB13 suggests possible recapitulation of developmental pathways in MEPN tumorigenesis. PDGFR alpha may be a potential therapeutic target in recurrent MEPN.
Figures



References
-
- (2001) FDA Oncology Tools Approval Summary for imatinib mesylate for Accel. Approv., U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa... (last accessed 15 September 2009).
-
- (2005) FDA Oncology Tools Approval Summary for sorafenib for the treatment of patients with advanced renal cell carcinoma. U.S. Food and Drug Administration Center for Drug Evaluation and Research. Available at: http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129234.htm (last accessed 15 September 2009).
-
- (2007) FDA Oncology Tools Approval Summary for sunitinib for the treatment of gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate; for the treatment of advanced renal cell carcinoma. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Available at: http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129255.htm (last accessed 15 September 2009).
-
- Bagley CA, Wilson S, Kothbauer KF, Bookland MJ, Epstein F, Jallo GI (2009) Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg Rev 32:321–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

