Safety and colonization of two novel VirG(IcsA)-based live Shigella sonnei vaccine strains in rhesus macaques (Macaca mulatta)
- PMID: 19793462
- PMCID: PMC2703165
Safety and colonization of two novel VirG(IcsA)-based live Shigella sonnei vaccine strains in rhesus macaques (Macaca mulatta)
Abstract
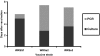
Shigella are gram-negative bacterium that cause bacillary dysentery (shigellosis). Symptoms include diarrhea and discharge of bloody mucoid stools, accompanied by severe abdominal pain, nausea, vomiting, malaise, and fever. Persons traveling to regions with poor sanitation and crowded conditions become particularly susceptible to shigellosis. Currently a vaccine for Shigella has not been licensed in the United States, and the organism quickly becomes resistant to medications. During the past 10 y, several live attenuated oral Shigella vaccines, including the strain WRSS1, have been tested in humans with considerable success. These Phase I vaccines lack the gene for the protein VirG also known as IcsA, which enables the organism to disseminate in the host target tissue. However, 5% to 20% of the vaccinated volunteers developed mild fever and brief diarrhea, and the removal of additional virulence-associated genes from the vaccine strain may reduce or eliminate these side effects. We administered 2 Shigella sonnei vaccines, WRSs2 and WRSs3, along with WRSS1 to compare their rates of colonization and clinical safety in groups of 5 rhesus macaques. The primate model provides the most physiologically relevant animal system to test the validity and efficacy of vaccine candidates. In this pilot study using a gastrointestinal model of infection, the vaccine candidates WRSs2 and WRSs3, which have additional deletions in the enterotoxin and LPS modification genes, provided better safety and comparable immunogenicity to those of WRSS1.
Figures
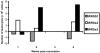
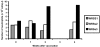
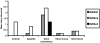
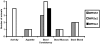
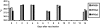

Similar articles
-
Shigella sonnei vaccine candidates WRSs2 and WRSs3 are as immunogenic as WRSS1, a clinically tested vaccine candidate, in a primate model of infection.Vaccine. 2011 Aug 26;29(37):6371-8. doi: 10.1016/j.vaccine.2011.04.115. Epub 2011 May 17. Vaccine. 2011. PMID: 21596086
-
Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity.Vaccine. 2010 Feb 10;28(6):1642-54. doi: 10.1016/j.vaccine.2009.11.001. Epub 2009 Nov 20. Vaccine. 2010. PMID: 19932216 Free PMC article.
-
Construction, characterization, and animal testing of WRSd1, a Shigella dysenteriae 1 vaccine.Infect Immun. 2002 Jun;70(6):2950-8. doi: 10.1128/IAI.70.6.2950-2958.2002. Infect Immun. 2002. PMID: 12010984 Free PMC article.
-
Shigellosis.J Microbiol. 2005 Apr;43(2):133-43. J Microbiol. 2005. PMID: 15880088 Review.
-
Shigellosis: the current status of vaccine development.Curr Opin Infect Dis. 2008 Jun;21(3):313-8. doi: 10.1097/QCO.0b013e3282f88b92. Curr Opin Infect Dis. 2008. PMID: 18448978 Review.
Cited by
-
Development of an Aotus nancymaae model for Shigella Vaccine immunogenicity and efficacy studies.Infect Immun. 2014 May;82(5):2027-36. doi: 10.1128/IAI.01665-13. Epub 2014 Mar 4. Infect Immun. 2014. PMID: 24595138 Free PMC article.
-
Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria.Front Immunol. 2013 Jul 4;4:176. doi: 10.3389/fimmu.2013.00176. eCollection 2013. Front Immunol. 2013. PMID: 23847617 Free PMC article.
-
NAIP-NLRC4-deficient mice are susceptible to shigellosis.Elife. 2020 Oct 19;9:e59022. doi: 10.7554/eLife.59022. Elife. 2020. PMID: 33074100 Free PMC article.
-
Shigella-mediated immunosuppression in the human gut: subversion extends from innate to adaptive immune responses.Hum Vaccin Immunother. 2019;15(6):1317-1325. doi: 10.1080/21645515.2019.1594132. Epub 2019 Apr 19. Hum Vaccin Immunother. 2019. PMID: 30964713 Free PMC article. Review.
-
BECC-engineered live-attenuated Shigella vaccine candidates display reduced endotoxicity with robust immunogenicity in mice.Res Sq [Preprint]. 2024 Jun 11:rs.3.rs-4448907. doi: 10.21203/rs.3.rs-4448907/v1. Res Sq. 2024. Update in: Vaccine. 2025 Mar 19;50:126779. doi: 10.1016/j.vaccine.2025.126779. PMID: 38946947 Free PMC article. Updated. Preprint.
References
-
- Banish LD, Sims R, Sack D, Montali RJ, Phillips L, Bush M. 1993. Prevalence of shigellosis and other enteric pathogens in a zoologic collection of primates. J Am Vet Med Assoc 203:126–132 - PubMed
-
- Beecham HJ, 3rd, Lebron CI, Echeverria P. 1997. Short report: impact of traveler's diarrhea on United States troops deployed to Thailand. Am J Trop Med Hyg 57:699–701 - PubMed
-
- Cossart P, Sansonetti PJ. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242–248 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
