Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles
- PMID: 19793818
- PMCID: PMC2786834
- DOI: 10.1128/JVI.01476-09
Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles
Erratum in
- J Virol. 2010 May;84(9):4864
Abstract
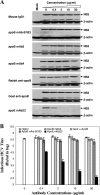
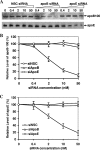
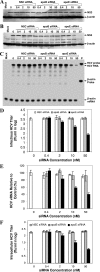
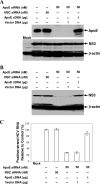
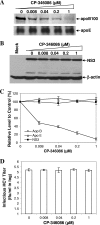
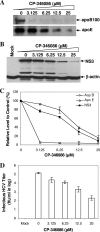
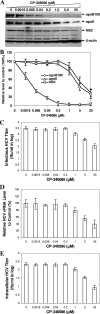
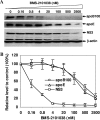
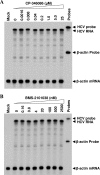
Our previous studies have found that hepatitis C virus (HCV) particles are enriched in apolipoprotein E (apoE) and that apoE is required for HCV infectivity and production. Studies by others, however, suggested that both microsomal transfer protein (MTP) and apoB are important for HCV production. To define the roles of apoB and apoE in the HCV life cycle, we developed a single-cycle HCV growth assay to determine the correlation of HCV assembly with apoB and apoE expression, as well as the influence of MTP inhibitors on the formation of HCV particles. The small interfering RNA (siRNA)-mediated knockdown of apoE expression remarkably suppressed the formation of HCV particles. However, apoE expressed ectopically could restore the defect of HCV production posed by the siRNA-mediated knockdown of endogenous apoE expression. In contrast, apoB-specific antibodies and siRNAs had no significant effect on HCV infectivity and production, respectively, suggesting that apoB does not play a significant role in the HCV life cycle. Additionally, two MTP inhibitors, CP-346086 and BMS-2101038, efficiently blocked secretion of apoB-containing lipoproteins but did not affect HCV production unless apoE expression and secretion were inhibited. At higher concentrations, however, MTP inhibitors blocked apoE expression and secretion and consequently suppressed the formation of HCV particles. Furthermore, apoE was found to be sensitive to trypsin digestion and to interact with NS5A in purified HCV particles and HCV-infected cells, as demonstrated by coimmunoprecipitation. Collectively, these findings demonstrate that apoE but not apoB is required for HCV assembly, probably via a specific interaction with NS5A.
Figures
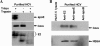
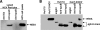
Similar articles
-
Amphipathic α-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles.PLoS Pathog. 2014 Dec 11;10(12):e1004534. doi: 10.1371/journal.ppat.1004534. eCollection 2014 Dec. PLoS Pathog. 2014. PMID: 25502789 Free PMC article.
-
Apolipoprotein E codetermines tissue tropism of hepatitis C virus and is crucial for viral cell-to-cell transmission by contributing to a postenvelopment step of assembly.J Virol. 2014 Feb;88(3):1433-46. doi: 10.1128/JVI.01815-13. Epub 2013 Oct 30. J Virol. 2014. PMID: 24173232 Free PMC article.
-
Hepatitis C Virus Strain-Dependent Usage of Apolipoprotein E Modulates Assembly Efficiency and Specific Infectivity of Secreted Virions.J Virol. 2017 Aug 24;91(18):e00422-17. doi: 10.1128/JVI.00422-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28659481 Free PMC article.
-
[Hepatic tropism of hepatitis C virus infection].Uirusu. 2018;68(1):63-70. doi: 10.2222/jsv.68.63. Uirusu. 2018. PMID: 31105136 Review. Japanese.
-
Lipids and HCV.Semin Immunopathol. 2013 Jan;35(1):87-100. doi: 10.1007/s00281-012-0356-2. Epub 2012 Oct 31. Semin Immunopathol. 2013. PMID: 23111699 Review.
Cited by
-
The Serum Very-Low-Density Lipoprotein Serves as a Restriction Factor against Hepatitis C Virus Infection.J Virol. 2015 Jul;89(13):6782-91. doi: 10.1128/JVI.00194-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903344 Free PMC article.
-
Exploitation of lipid components by viral and host proteins for hepatitis C virus infection.Front Microbiol. 2012 Feb 14;3:54. doi: 10.3389/fmicb.2012.00054. eCollection 2012. Front Microbiol. 2012. PMID: 22347882 Free PMC article.
-
Hepatitis C virus infection and tight junction proteins: The ties that bind.Biochim Biophys Acta Biomembr. 2020 Jul 1;1862(7):183296. doi: 10.1016/j.bbamem.2020.183296. Epub 2020 Apr 5. Biochim Biophys Acta Biomembr. 2020. PMID: 32268133 Free PMC article. Review.
-
Modulation of host lipid metabolism by hepatitis C virus: Role of new therapies.World J Gastroenterol. 2015 Oct 14;21(38):10776-82. doi: 10.3748/wjg.v21.i38.10776. World J Gastroenterol. 2015. PMID: 26478669 Free PMC article. Review.
-
Hepatitis C virus relies on lipoproteins for its life cycle.World J Gastroenterol. 2016 Feb 14;22(6):1953-65. doi: 10.3748/wjg.v22.i6.1953. World J Gastroenterol. 2016. PMID: 26877603 Free PMC article. Review.
References
-
- Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6-S9. - PubMed
-
- Andre, P., G. Perlemuter, A. Budkowska, C. Brechot, and V. Lotteau. 2005. Hepatitis C virus particles and lipoprotein metabolism. Semin. Liver Dis. 25:93-104. - PubMed
-
- Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

