Acute infection with venezuelan equine encephalitis virus replicon particles catalyzes a systemic antiviral state and protects from lethal virus challenge
- PMID: 19793821
- PMCID: PMC2786707
- DOI: 10.1128/JVI.00564-09
Acute infection with venezuelan equine encephalitis virus replicon particles catalyzes a systemic antiviral state and protects from lethal virus challenge
Abstract
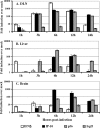
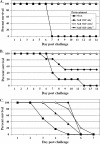
The host innate immune response provides a critical first line of defense against invading pathogens, inducing an antiviral state to impede the spread of infection. While numerous studies have documented antiviral responses within actively infected tissues, few have described the earliest innate response induced systemically by infection. Here, utilizing Venezuelan equine encephalitis virus (VEE) replicon particles (VRP) to limit infection to the initially infected cells in vivo, a rapid activation of the antiviral response was demonstrated not only within the murine draining lymph node, where replication was confined, but also within distal tissues. In the liver and brain, expression of interferon-stimulated genes was detected by 1 to 3 h following VRP footpad inoculation, reaching peak expression of >100-fold over that in mock-infected animals. Moreover, mice receiving a VRP footpad inoculation 6, 12, or 24 h prior to an otherwise lethal VEE footpad challenge were completely protected from death, including a drastic reduction in challenge virus titers. VRP pretreatment also provided protection from intranasal VEE challenge and extended the average survival time following intracranial challenge. Signaling through the interferon receptor was necessary for antiviral gene induction and protection from VEE challenge. However, VRP pretreatment failed to protect mice from a heterologous, lethal challenge with vesicular stomatitis virus, yet conferred protection following challenge with influenza virus. Collectively, these results document a rapid modulation of the host innate response within hours of infection, capable of rapidly alerting the entire animal to pathogen invasion and leading to protection from viral disease.
Figures




References
-
- Alsharifi, M., M. Regner, R. Blanden, M. Lobigs, E. Lee, A. Koskinen, and A. Mullbacher. 2006. Exhaustion of type I interferon response following an acute viral infection. J. Immunol. 177:3235-3241. - PubMed
-
- Anishchenko, M., S. Paessler, I. P. Greene, P. V. Aguilar, A.-S. Carrara, and S. C. Weaver. 2004. Generation and characterization of closely related epizootic and enzootic infectious cDNA clones for studying interferon sensitivity and emergence mechanisms of Venezuelan equine encephalitis virus. J. Virol. 78:1-8. - PMC - PubMed
-
- Aronson, J. F., F. B. Grieder, N. L. Davis, P. C. Charles, T. Knott, K. Brown, and R. E. Johnston. 2000. A single-site mutant and revertants arising in vivo define early steps in the pathogenesis of Venezuelan equine encephalitis virus. Virology 270:111-123. - PubMed
-
- Barna, M., T. Komatsu, Z. Bi, and C. S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31-39. - PubMed
-
- Bennett, A. M., S. J. Elvin, A. J. Wright, S. M. Jones, and R. J. Phillpotts. 2000. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine 19:337-347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

