Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5
- PMID: 19793826
- PMCID: PMC2786738
- DOI: 10.1128/JVI.01593-09
Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5
Abstract
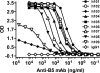
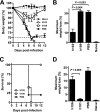
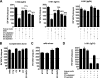
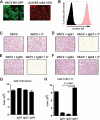
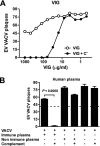
Antibodies against the extracellular virion (EV or EEV) form of vaccinia virus are an important component of protective immunity in animal models and likely contribute to the protection of immunized humans against poxviruses. Using fully human monoclonal antibodies (MAbs), we now have shown that the protective attributes of the human anti-B5 antibody response to the smallpox vaccine (vaccinia virus) are heavily dependent on effector functions. By switching Fc domains of a single MAb, we have definitively shown that neutralization in vitro--and protection in vivo in a mouse model--by the human anti-B5 immunoglobulin G MAbs is isotype dependent, thereby demonstrating that efficient protection by these antibodies is not simply dependent on binding an appropriate vaccinia virion antigen with high affinity but in fact requires antibody effector function. The complement components C3 and C1q, but not C5, were required for neutralization. We also have demonstrated that human MAbs against B5 can potently direct complement-dependent cytotoxicity of vaccinia virus-infected cells. Each of these results was then extended to the polyclonal human antibody response to the smallpox vaccine. A model is proposed to explain the mechanism of EV neutralization. Altogether these findings enhance our understanding of the central protective activities of smallpox vaccine-elicited antibodies in immunized humans.
Figures
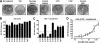
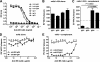
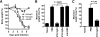
Comment in
-
Complement-bound human antibodies to vaccinia virus B5 antigen protect mice from virus challenge.Expert Rev Vaccines. 2010 Mar;9(3):255-9. doi: 10.1586/erv.10.5. Expert Rev Vaccines. 2010. PMID: 20218853 Free PMC article.
References
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. - PMC - PubMed
-
- Aldaz-Carroll, L., Y. Xiao, J. C. Whitbeck, M. P. de Leon, H. Lou, M. Kim, J. Yu, E. L. Reinherz, S. N. Isaacs, R. J. Eisenberg, and G. H. Cohen. 2007. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J. Virol. 81:8131-8139. - PMC - PubMed
-
- Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320-337. - PubMed
-
- Angal, S., D. J. King, M. W. Bodmer, A. Turner, A. D. Lawson, G. Roberts, B. Pedley, and J. R. Adair. 1993. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol. Immunol. 30:105-108. - PubMed
-
- Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

