Development of multisensory convergence in the Xenopus optic tectum
- PMID: 19793878
- PMCID: PMC2804420
- DOI: 10.1152/jn.00632.2009
Development of multisensory convergence in the Xenopus optic tectum
Abstract
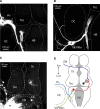
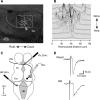
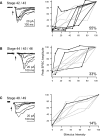
The adult Xenopus optic tectum receives and integrates visual and nonvisual sensory information. Nonvisual inputs include mechanosensory inputs from the lateral line, auditory, somatosensory, and vestibular systems. While much is known about the development of visual inputs in this species, almost nothing is known about the development of mechanosensory inputs to the tectum. In this study, we investigated mechanosensory inputs to the tectum during critical developmental stages (stages 42-49) in which the retinotectal map is being established. Tract-tracing studies using lipophilic dyes revealed a large projection between the hindbrain and the tectum as early as stage 42; this projection carries information from the Vth, VIIth, and VIIIth nerves. By directly stimulating hindbrain and visual inputs using an isolated whole-brain preparation, we found that all tectal cells studied received both visual and hindbrain input during these early developmental stages. Pharmacological data indicated that the hindbrain-tectal projection is glutamatergic and that there are no direct inhibitory hindbrain-tectal ascending projections. We found that unlike visual inputs, hindbrain inputs do not show a decrease in paired-pulse facilitation over this developmental period. Interestingly, over this developmental period, hindbrain inputs show a transient increase followed by a significant decrease in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA)/N-methyl-D-aspartate (NMDA) ratio and show no change in quantal size, both in contrast to visual inputs. Our data support a model by which fibers are added to the hindbrain-tectal projection across development. Nascent fibers form new synapses with tectal neurons and primarily activate NMDA receptors. At a time when retinal ganglion cells and their tectal synapses mature, hindbrain-tectal synapses are still undergoing a period of rapid synaptogenesis. This study supports the idea that immature tectal cells receive converging visual and mechanosensory information and indicates that the Xenopus tectum might be an ideal preparation to study the early development of potential multisensory interactions at the cellular level.
Figures





References
-
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol 97: 2949–2957, 2007 - PubMed
-
- Akaneya Y, Altinbaev R, Bayazitov IT, Kinoshita S, Voronin LL, Tsumoto T. Low-frequency depression of synaptic responses recorded from rat visual cortex. Neuroscience 117: 305–320, 2003 - PubMed
-
- Behrend O, Branoner F, Zhivkov Z, Ziehm U. Neural responses to water surface waves in the midbrain of the aquatic predator Xenopus laevis laevis. Eur J Neurosci 23: 729–744, 2006 - PubMed
-
- Chahoud BH, Cordier-Picouet MJ, Clairambault P. Larval development of tectal efferents and afferents in Xenopus laevis (Amphibia Anura). J Hirnforsch 37: 519–535, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

